Abstract
Objective:
Laparoscopic and robotic sacrocolpopexy are widely used for pelvic organ prolapse (POP) treatment. Evidence comparing outcomes and costs is lacking. We compared costs and clinically relevant outcomes in women randomized to laparoscopic sacrocolpopexy compared with robotic sacrocolpopexy.
Methods:
Participants with symptomatic stage POP II or greater, including significant apical support loss, were randomized to either laparoscopic or robotic sacrocolpopexy. We compared surgical costs (including costs for robot, initial hospitalization) and re-hospitalization within 6 weeks. Secondary outcomes included postoperative pain, POP quantification, symptom severity and quality of life, and adverse events.
Results:
We randomized 78 women [mean age 59 years]: laparoscopic (n=38), robotic (n=40). The robotic sacrocolpopexy group had higher initial hospital costs ($19,616 vs. $11,573, p < 0.001) and over 6 weeks, hospital costs remained higher for robotic sacrocolpopexy ($20,898 vs. $12,170, p < 0.001). When we excluded costs of robot purchase and maintenance, we did not detect a statistical difference in initial day of surgery costs of robotic vs. laparoscopic ($12,586 vs. $11,573; p = 0.160) or hospital costs over 6 weeks ($13,867 vs. $12,170; p = 0.060). The robotic group had longer operating room times (202.8 min vs. 178.4 min, p = 0.030) and higher pain scores 1-week after surgery (3.5 ± 2.1 vs. 2.6 ± 2.2; p = 0.044). There were no group differences in symptom bother by Pelvic Floor Distress Inventory, POP stage, or rate of adverse events.
Conclusion:
Costs of robotic sacrocolpopexy are higher than laparoscopic, while short-term outcomes and complications are similar. Primary cost differences resulted from robot maintenance and purchase costs.
Introduction
The abdominal sacrocolpopexy is considered to be a gold standard in surgical treatment of apical vaginal prolapse (1,2,3). Minimally invasive approaches specifically reduce morbidity associated with open sacrocolpopexy, facilitating patient recovery. Laparoscopic sacrocolpopexy has similar outcomes to abdominal sacrocolpopexy, but is technically challenging such that it is usually performed by expert laparoscopists (3). When compared to open techniques, robotic abdominal sacrocolpopexy is associated with less blood loss, shorter lengths of stay, and longer operative times (1, 2). Robotic technology has made laparoscopic sacrocolpopexy a more feasible procedure for many pelvic surgeons because the improved dexterity of the robot and precision of instruments allow suturing of mesh to the vagina to be accomplished with ease. Further, the three-dimensional imaging of the robotic camera provides close visualization of the vessels overlying the sacral promontory, and may allow for better preservation of these vessels and potentially less blood loss. This technology may therefore affect learning curves such that fewer cases are needed for a surgeon to gain competence.
It is not known whether costs or patient outcomes differ between laparoscopic and robotic, as studies primarily comparing cost outcomes of robotic and laparoscopic are lacking. As part of the American Recovery and Reinvestment Act (ARRA) in 2009, the National Institutes of Health distributed a request for applications addressing the Specific Challenge Topic: Comparative Effectiveness of Robotic Surgery (05-EB-104). We sought to address this ARRA goal by directly comparing costs and relevant secondary outcomes between laparoscopic and robotic in two surgical centers (NCT01124916).
Materials and Methods
Abdominal Colpopexy: Comparison of Endoscopic Surgical Strategies (ACCESS) is a randomized comparative effectiveness trial conducted at UCLA/Cedars-Sinai and Loyola University Medical Centers, with Institutional Review Board approval obtained at each site. The primary aim was to compare costs of robotic and laparoscopic. Detailed methods are described in a separate article by Mueller et al (4). Entry criteria included a clinical indication for sacrocolpopexy in women with symptomatic stage II or greater pelvic organ prolapse (POP), with the leading edge of the prolapse to 1 cm on either side of the introitus, including apical support loss to ½ total vaginal length (the top of the vagina or cervix descending down at least halfway down the vaginal canal). After research consent, participants were randomized to laparoscopic or robotic sacrocolpopexy on the day of surgery. Surgeons were required to have performed at least 10 procedures of each type prior to study participation. Sacrocolpopexy was performed with two separate pieces of synthetic mesh (vs. Y-shaped mesh) and Gore-Tex sutures (4). Surgeon preference determined the brand of mesh used and whether the retroperitoneal lining was re-approximated after mesh tensioning. Colpopexy techniques did not vary by randomization arm. Concomitant surgeries, including hysterectomy, posterior repair, and retropubic synthetic midurethral slings were allowed.
Healthcare costs were assessed from the healthcare provider’s (i.e., hospital and physician’s) perspectives and included costs of the hospital and physician services, costs of the robot and its maintenance, and costs of disposable instruments for both treatment arms. To estimate hospital costs, we obtained charges from each patient’s billing information and then applied cost-to-charge ratios. The cost-to-charge ratios for each facility were obtained from the cost reports the hospitals submit annually to the Centers for Medicare & Medicaid Services (CMS). Estimates of physician costs were based on billing information (e.g., charges on the CMS 1500 form or equivalent). A cost per procedure for the robot was estimated based on the average purchase price of the robots at each facility, the number of years a robot will provide service, the resale/trade-in value, the annual maintenance costs of the robots, and the number of treatments for which a robot is used per year across all services that used a robot. Costs of subsequent re-hospitalizations during 6 weeks after discharge for the procedure were estimated from charges recorded on uniform billing 2004 (UB-04) forms or equivalent, which were converted to costs using facility-specific cost-to-charge ratios, and physician costs were based on billing information. All costs associated with surgical procedure including costs for robot, initial hospitalization, and any re-hospitalization in the 1st 6 weeks were compared between groups. To determine the effect that the cost of purchasing and maintaining the robot had on our results, we calculated costs both including and excluding the cost of the robot. Additionally, to determine the effect that concomitant procedures (e.g., hysterectomy, posterior repair, and midurethral sling) had on our results, we stratified the analyses according to whether the patient had a concomitant procedure. Secondary outcomes included postoperative pain, POP quantification, symptom severity and quality of life, and adverse events (AE). A priori power calculation determined that 32 women in each arm would provide 95% power to detect a difference of at least $2,500 difference in total charges, using a 2-sided t-test with a 0.05 significance level and standard deviations similar to previously published work (4,5). Additional patients would be recruited until the end of the study period to account for any loss to follow-up.
After enrollment, the following baseline and follow-up data were obtained at scheduled intervals: demographic information, medical history and medications, physical examination, including pelvic organ prolapse quantification (POPQ) measurements, the Brinks scale of pelvic muscle strength, and an assessment of vaginal integrity (6, 7). General health-related quality of life was assessed with the Short Form Health Survey (SF-36) (8) and the EuroQol-5D (EQ-5D) (9). Quality-of-life instruments specific to pelvic floor disorders included the Patient Global Impression of Improvement (PGI-I), the Hunskaar Severity Index, the Pelvic Floor Distress Inventory (PFDI), the Pelvic Floor Impact Questionnaire (PFIQ) (10, 11), and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) (12). Patient convalescence was measured using the Activities Assessment Scale (AAS) (13), as well as the Convalescence and Recovery Evaluation (CARE) (14). Pain levels were measured with validated Surgical Pain Scales (SPS) scores (15).
Quality-adjusted life years (QALYs) are a measure of health-related quality of life that takes into account both the quantity and quality of life. QALYs were calculated using information obtained from the EQ-5D, a validated survey instrument that measures health status. The EQ-5D was collected at baseline, and 2 and 6 weeks after surgery. Each patient’s responses to the EQ-5D were converted to a utility weight based on U.S. population preferences. Utility weights from the EQ-5D are on a scale where 0.0 = death and 1.0 = perfect health. Following prior studies, QALYs were calculated assuming linear changes in each subject’s utility weights over time and calculating the area under the curve over the 2-week and 6-week periods.
Means and standard deviations (SD) or counts and percentages were computed for continuous and categorical data, respectively. Differences in group means were tested by way of a Student’s t-test, or a Wilcoxon rank sum test where data failed to follow a normal distribution after testing with the Kolmogorov-Smirnov test for normality. Differences in group proportions were tested by way of a Chi-square test, or a Fisher’s exact test where data were sparse. Differences were considered statistically different where p < 0.05. The frequency and severity of adverse events was scored by means of an indexed summary score (16) and tested across treatment groups by way of a Student’s t-test. Due to the exploratory nature of these secondary outcomes, no adjustments were made for repeated measurements. All analyses were performed using SAS v9.3.
Results
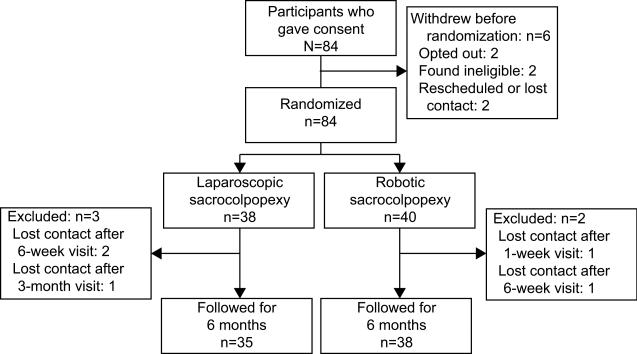
We randomized 78 women [mean age 59 years (range 26-79): laparoscopic (n=38) and robotic (n=40). Only three patients (1 laparoscopic, 2 robotic) were lost to follow-up prior to the 6-month visit (Figure 1). Table 1 depicts the baseline demographic and comorbidities and concomitant procedures. Participants were predominantly non-Hispanic and Caucasian (87%). Rate of concomitant procedures, including supracervical hysterectomy, sling, and anterior and posterior repairs, was similar between groups as were baseline POPQ measurements and PFDI/PFIQ scores. There was no difference in the frequencies by which each of the five surgeons were randomized to a given arm (p=0.3379).
Figure 1.
Diagram showing flow of participants.
Table 1.
Baseline Characteristics
| All Cases | Laparoscopic | Robotic | P | ||||
|---|---|---|---|---|---|---|---|
| Age | 59.5 | (9.9) | 60.6 | (9.2) | 58.5 | (10.5) | 0.351 |
| BMI | 27.7 | (5.7) | 27 | (4.7) | 28.2 | (6.6) | 0.292 |
| Parity | 2.7 | (1.2) | 2.7 | (1.3) | 2.6 | (1.1) | 0.723 |
| Study site | |||||||
| Loyola | 56 | 72% | 28 | 74% | 28 | 70% | 0.804 |
| UCLA/Cedars-Sinai | 22 | 28% | 10 | 26% | 12 | 30% | |
| More than high school education | 50 | 64% | 24 | 63% | 26 | 65% | 0.999 |
| Race | |||||||
| African American | 5 | 6% | 1 | 3% | 4 | 10% | 0.676 |
| Caucasian | 71 | 91% | 36 | 95% | 35 | 88% | |
| Other | 2 | 3% | 1 | 3% | 1 | 3% | |
| Hispanic ethnicity | 4 | 5% | 2 | 5% | 2 | 5% | 0.999 |
| Household income | |||||||
| < $50,000 | 29 | 37% | 18 | 47% | 11 | 28% | 0.148 |
| $50,000 to $75,000 | 22 | 28% | 11 | 29% | 11 | 28% | |
| $75,000 or more | 23 | 30% | 8 | 21% | 15 | 38% | |
| Major comorbidities | |||||||
| Diabetes | 9 | 12% | 3 | 8% | 6 | 15% | 0.482 |
| Heart attack | 6 | 8% | 1 | 3% | 5 | 13% | 0.201 |
| Stroke | 2 | 3% | 2 | 5% | 0 | 0% | 0.234 |
| Asthma, emphysema | 16 | 21% | 6 | 16% | 10 | 25% | 0.404 |
| Cancers | 14 | 18% | 6 | 16% | 8 | 20% | 0.628 |
| Stomach ulcer, IBS | 17 | 22% | 7 | 18% | 10 | 25% | 0.587 |
| Postmenopausal | 58 | 74% | 29 | 76% | 29 | 73% | 0.798 |
| Current estrogen therapy (local or systemic) | 17 | 22% | 11 | 29% | 6 | 15% | 0.174 |
| Previous surgery for UI | 12 | 15% | 7 | 18% | 5 | 13% | 0.541 |
| Previous surgery for POP | 16 | 21% | 8 | 21% | 8 | 20% | 0.999 |
| Prior Hysterectomy | 33 | 42% | 19 | 50% | 14 | 35% | 0.252 |
| Concurrent procedures at surgery | |||||||
| Hysterectomy | 45 | 58% | 20 | 53% | 25 | 63% | 0.492 |
| Retropubic midurethral sling | 47 | 60% | 21 | 55% | 26 | 65% | 0.488 |
| Anterior or posterior repair | 5 | 6% | 4 | 11% | 1 | 3% | 0.195 |
BMI, body mass index; IBS, irritable bowel syndrome; UI, urinary incontinence; POP, pelvic organ prolapse. Data are mean±standard deviation or n(%) unless otherwise specified.
Overall laparoscopic procedure time recorded as time to first incision to time undocking (robotic group) or time of last suspension suture (laparoscopic group) was an average of 24.4 minutes longer in the robotic arm (202 vs. 179 minutes, p = 0.030, Table 2), but total surgery time did not vary by treatment arm. Though specific docking times were not recorded, the longer time in the robotic arm is attributable to both time docking the robot and console time. Blood loss and intraoperative complications were not statistically different between groups. In laparoscopic arm, 10 unique AE were reported (4 Dindo II, 6 Dindo III) with 6 AE in robotic arm (1 Dindo I, 2 Dindo II, 2 Dindo III, 1 Dindo IV). Based on an indexed value of severity and number of AE (14), there was no significant group difference (p = 0.868). AE’s consisted of one left iliac venotomy in each arm (closed intraoperatively without changing route of access), and one small bowel obstruction in each arm (one in the robotic arm requiring surgical exploration). Additionally, in the laparoscopic arm there were three cases of vaginal granulation tissue and suture exposure, a port site herniation requiring re-operation, and one cystotomy identified and closed intraoperatively. Non-technical complications included a pulmonary embolism (robotic), an episode of atrial fibrillation requiring ablation (laparoscopic), and hematemesis in one patient (laparoscopic).
Table 2.
Cost and Surgical Outcomes
| Laparoscopic (n=38) | Robotic (n=40) | P | |||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | ||
| Blood loss (cc) | |||||
| Abdominal sacrocolpopexy |
60.0 | (208.5) | 41.3 | (37.0) | 0.113 |
| Total | 106.4 | (206.9) | 85.1 | (51.9) | 0.303 |
| Times (min) | |||||
| Procedure | 178.4 | (49.8) | 202.8 | (46.1) | 0.030 |
| Total surgery | 225.5 | (62.3) | 246.5 | (51.3) | 0.110 |
| Day of surgery | |||||
| Costs | |||||
| Excluding robotics |
$11,573 | ($3,191) | $12,586 | ($3,135) | 0.160 |
| Including robotics | $11,573 | ($3,191) | $19,616 | ($3,135) | <0.001* |
| Total 6-week costs | |||||
| Excluding robotics |
$12,170 | ($4,129) | $13,867 | ($3,386) | 0.060 |
| Including robotics | $12,170 | ($4,129) | $20,898 | ($3,386) | <0.001* |
SD, standard deviation.
Difference is significant with bias-corrected bootstrap analysis with 2,000 replications.
The average purchase price of a robot at each site was $1,838,140, and annual maintenance costs were $153,000/yr. The number of uses per year per robot averaged 300 across study sites. Accounting for an annuity factor and resale value and trade-in of $200,000 for 7 years of use, the equivalent annual cost was $7,030 per procedure. Initial day of surgery hospital costs for robotic sacrocolpopexy were higher than for laparoscopic ($19,616 vs. $11,573; p < 0.001, Table 2). Over 6 weeks, hospital costs remained higher for robotic ($20,898 vs. $12,170; p = <0.001. When we excluded costs of robot purchase and maintenance, we did not detect a statistical difference in initial day of surgery costs of robotic vs. laparoscopic ($12,586 vs. $11,573; p = 0.160) or hospital costs over 6 weeks ($13,867 vs. $12,170; p = 0.060). When the actual purchase and maintenance cost of the robotic were excluded the cost difference between robotic and laparoscopic sacrocolpopexy decreased to only $1000. Additionally, when we stratified by whether or not patients had a concomitant procedure, costs of the initial day of the surgery were higher in the robotic than the laparoscopic group for the 33 patients without a concomitant hysterectomy ($18,169 vs. $10,087; p < 0.001) when the robot was included, but were similar when the robot costs were excluded ($11,138 vs. $10,087; p = 0.281). A similar pattern existed for the 45 patients with a concomitant hysterectomy; costs of the initial day of the surgery were higher in the robotic than the laparoscopic group ($20,485 vs. $12,910; p < 0.001) when the robot was included but were similar when the robot costs were excluded ($13,454 vs. $12,910; p = 0.552). When the costs of the initial day of surgery were stratified by the 67 patients with and the 11 patients without any concomitant procedure, the costs were significantly higher in the robotic group than the laparoscopic group, but there was no significant difference without the robot costs. A similar pattern also emerged for hospital costs over 6-weeks; costs were higher in the robotic than laparoscopic groups when the robot costs were included, but were similar when the robot costs were excluded for the 67 patients with and 11 patients without any concomitant procedures, as well as for the 45 patients with a concomitant hysterectomy. However, among the 33 patients without a concomitant hysterectomy, hospital costs over 6 weeks were higher in the robotic than laparoscopic group when the robot costs were included or excluded, due to two rehospitalizations in the robotic but no rehospitalizations in laparoscopic group.
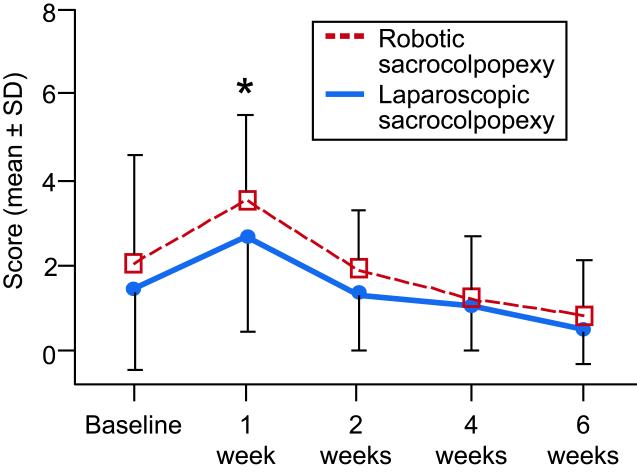
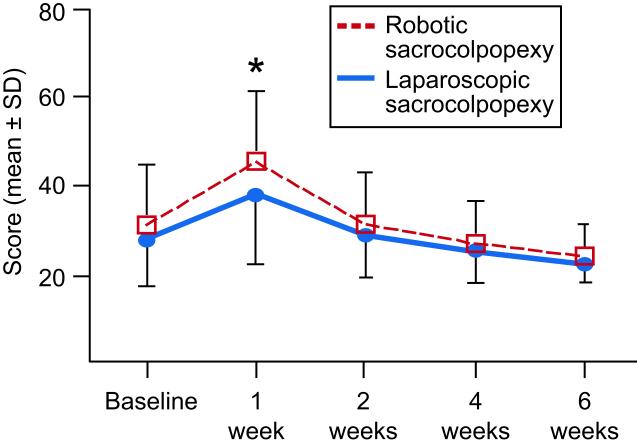
At one week after surgery, robotic patients reported more pain at normal activities (robotic: 3.5 ± 2.1; laparoscopic: 2.6 ± 2.2; p = 0.044, Figure 2), more unpleasantness of the worst pain (robotic: 2.4 ± 2.0; laparoscopic: 1.8 ± 1.5; p = 0.026), and more difficulty with activities (robotic: 45.4 ± 16.1; laparoscopic: 38.1 ± 15.5; p = 0.039, Figure 3). These differences between groups were all diminished by 2 weeks after surgery. The SF-36 Physical Health score was worse in the robotic arm at 2 weeks (robotic: 41.0 ± 26.0; laparoscopic: 57.1 ± 25.6; p = 0.009), but no differences were observed between groups by the 6-week time point.
Figure 2.
Surgical Pain Scale scores at normal activities for laparoscopic sacrocolpopexy and robotic sacrocolpopexy groups. Scores are rated from 0 (no pain) to 10 (worse possible pain). (*Indicates significant difference, p < 0.05) SD, standard deviation.
Figure 3.
Activities Assessment Scale scores for laparoscopic sacrocolpopexy and robotic sacrocolpopexy groups. Scores are rated from 0 (no difficulty) to 100 (unable). (*Indicates significant difference, p < 0.05) SD, standard deviation.
At 6 months, there was no difference between groups with respect to clinical outcomes, including POPQ measurements and subscales of the PFDI and PFIQ (Table 3). Nine women in the laparoscopic arm and five from the robotic arm had recurrent UI symptoms (p = 0.198). Three women in the laparoscopic arm and two in the robotic underwent later sling surgery for SUI within one year of the colpopexy (p = 0.601).
Table 3.
Clinical Outcomes
|
Laparoscopic
(n=38) |
Robotic
(n=40) |
Treatmen t Effect P |
|||||
|---|---|---|---|---|---|---|---|
| Baselin e |
3 Months |
6 Months |
Baselin e |
3 Months |
6 Months |
||
| POP-Q (cm) Point Ba |
2.45 | −2.34 | −2.43 | 2.58 | −2.56 | −2.48 | 0.833 |
| (1.84) | (1.19) | (0.86) | (2.01) | (0.69) | (0.76) | ||
| POP-Q (cm) Point C |
0.74 | −7.74 | −7.30 | 0.25 | −7.89 | −7.97 | 0.502 |
| (3.64) | (4.74) | (6.04) | (3.98) | (5.05) | (4.37) | ||
| POPQ (cm) Point Bp |
0.21 | −2.66 | −2.63 | −0.50 | −2.17 | −2.33 | 0.977 |
| (3.05) | (0.59) | (0.67) | (2.94) | (1.48) | (1.22) | ||
| UDI* | 97.5 | 25.7 | 25.1 | 110.1 | 30.3 | 31.3 | 0.208 |
| (60.4) | (40.8) | (31.4) | (58.7) | (42.1) | (35.3) | ||
| POPDI* | 116.5 | 28.7 | 22.6 | 126.6 | 32.7 | 34.8 | 0.177 |
| (60.8) | (28.3) | (25.9) | (63.1) | (45.4) | (41.0) | ||
| CRADI* | 99.0 | 34.3 | 34.8 | 90.1 | 44.1 | 43.4 | 0.756 |
| (71.7) | (33.5) | (44.9) | (71.9) | (48.3) | (49.1) | ||
| UIQ* | 97.6 | 31.1 | 31.8 | 128.3 | 29.4 | 20.6 | 0.501 |
| (96.3) | (71.1) | (57.8) | (93.8) | (56.6) | (43.3) | ||
| CRAIQ* | 67.5 | 17.2 | 24.1 | 67.0 | 20.8 | 17.3 | 0.881 |
| (87.5) | (33.8) | (52.4) | (89.8) | (38.3) | (34.3) | ||
| POPIQ* | 83.2 | 17.2 | 9.4 | 114.4 | 14.7 | 14.6 | 0.181 |
| (83.7) | (59.7) | (36.1) | (102.4) | (33.5) | (39.4) | ||
POP-Q, pelvic organ prolapse quantification; UDI, Urinary Distress Inventory; POPDI, Pelvic Organ Prolapse Distress Inventory; CRADI, Colon Rectal Anal Distress Inventory; UIQ, Urinary Impact Questionnaire; CRAIG, Colon Rectal Anal Impact Questionnaire; POPIQ, Pelvic Organ Prolapse Impact Questionnaire.
UDI scores range from 0−300, POPDI scores range from 0−300, CRADI scores range from 0−400, UIQ scores range from 0−400, CRAIQ scores range from 0−400, POPIQ scores range from 0−400, and PFDI and PFIQ subscale scores range from 0−400 with higher scores indicating worsening symptoms. Data are mean±standard deviation unless otherwise specified.
EQ-5D scores were not significantly different between groups both at baseline (laparoscopic: 0.86 ± 0.12; robotic: 0.83 ± 0.15; p = 0.374) and at 6 weeks after surgery (laparoscopic: 0.91 ± 0.11; robotic: 0.90 ± 0.10; p= 0.685). QALYs at six weeks after surgery were not significantly different between the two arms, 0.101 ± 0.009 in the laparoscopic and 0.098 ± 0.011 in the robotic arm (p = 0.234).
Discussion
This study, which reports prospectively collected cost data from a randomized comparative effectiveness trial, demonstrates that increased cost of robotic surgery is due to the robot purchase and maintenance, rather than surgical costs. Numerous cost studies published have used retrospective data or disease simulation models in which the investigator makes assumptions regarding costs and outcomes to compare robotic, open, and laparoscopic procedures (17-23). Though most studies agree that prolapse outcomes are similar, the conclusions of these studies vary tremendously based on model assumptions and analyses.
Some peer-reviewed literature concludes that robotic sacrocolpopexy is equally or less costly than open abdominal sacrocolpopexy (18, 19). Taking hospital stay into account, a retrospective study by Hoyte et al found costs of robotic to be less than the open approach (18). Elliott et al found in a retrospective cohort that robotic is equally or less costly than an open approach, but this finding is dependent on a sufficient robotic case volume and a shorter stay in the robotic arm at a given institution (19). Other work, also retrospective, has demonstrated that robotic is more expensive than laparoscopic or open abdominal sacrocolpopexy (17, 21). Tan-Kim et al found that costs were higher in the robotic arm, though direct costs for hospital stay were similar. The increased costs in the robotic group was attributable to increased operative time (281 +/− 58 min. robotic vs. 206 +/− 42 minutes in laparoscopic arm, p < 0.001) (15). Our work showed a relatively small increase in operative time in the robotic group (24.4 minutes), but this did not translate into additional costs. A cost-minimization analysis by Judd et al concluded that robotic was more expensive than laparoscopic or open abdominal sacrocolpopexy under baseline assumptions (21). In a model in which a robot was already present at a given hospital, robotic sacrocolpopexy cost $8508 per procedure, and laparoscopic sacrocolpopexy cost $7353, a difference of $1,155 per case. We identified a similar cost difference between robotic and laparoscopic sacrocolpopexy excluding robot purchase price (12,586 vs. $11573, a difference of $1,013), but this difference did not reach statistical significance. Our costs were higher than those in the model by Judd et al because we included all costs for the day of surgery.
Both procedures were associated with low levels of postoperative pain. However, patient who underwent robotic surgery experienced more pain and a slower recovery to normal activities. Consistent with prior studies (24), pain in the first week after surgery was higher after robotic compared to laparoscopic; however, this difference in pain resolved by the second postoperative week. Paraiso et al randomized patients with posthysterectomy vaginal vault prolapse to robotic and laparoscopic sacrocolpopexy. Patients in the robotic arm had significantly higher pain at rest and with activity during weeks 3-5 after surgery, and required significantly more anti-inflammatory pain medication than the laparoscopic arm (24). The consistency in our findings with other investigators suggests a robotic approach may be associated with slightly higher postoperative pain, although the clinical significance of the increased pain scores in our study is questionable given that it translates into an approximately 1 point increase on a numeric rating scale. We suspect this temporary increase in pain may be a result of increased tension placed on robotic ports, likely from a lack of tactile feedback in that the surgeon is unable to sense the pressure placed on the ports. Increased surgical time may also be a factor contributing to pain in the robotic arm (24).
Although our study was powered on cost rather than prolapse outcomes, it does appear that outcomes at 6 months were similar, attesting to the ability of laparoscopic, including robotic, approaches to achieve success in cure of prolapse in a minimally invasive fashion. Although inclusion of an open arm would contribute significantly to our analyses, randomizing patients to an open arm, when given the option of a minimally invasive approach, would be difficult. Our study did not access the feasibility and applicability of laparoscopic and robotic approaches to surgeons in non-academic medical centers. All study surgeons were skilled in both robotic and laparoscopic sacrocolpopexy, limiting the generalizability of these data to surgeons who may be more experienced in one technique or the other. Though a requirement of only 10 cases per surgeon per technique should have excluded surgeons who were still learning, it is possible that more cases are needed to overcome the learning curve. Since our study included only two sites, we were not powered to measure the effects of surgeon volume on operative time and cost. In addition, results of our study may not be relevant for surgeons who, based on skill and preference, prefer robotic techniques. Similarly, all procedures were done in academic centers with resident, fellow, or resident and fellow participation, a factor that significantly contributes to operative time.
Despite similar clinical outcomes for prolapse, robotic sacrocolpopexy is associated with higher costs than laparoscopic sacrocolpopexy and is associated with minor clinical differences that may slow recovery.
Acknowledgments
Funded by a National Institute of Biomedical Imaging and Bioengineering Recovery Act Limited Competition Challenge Grant (1 RC1 EB010649-01).
The authors thank Thea Rogers, MA, for her work with programming and analysis.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Clinical Trial Registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT01124916.
References
- 1.Geller EJ, Siddiqui NY, Wu JM, Visco AG. Short-term outcomes of robotic sacrocolpopexy compared with abdominal sacrocolpopexy. Obstet Gynecol. 2008;112(6):1201–6. doi: 10.1097/AOG.0b013e31818ce394. [DOI] [PubMed] [Google Scholar]
- 2.McDermott CD, Hale DS. Abdominal, laparoscopic, and robotic surgery for pelvic organ prolapse. Obstet Gynecol Clin North Am. 2009;36(3):585–614. doi: 10.1016/j.ogc.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Pollard ME, Eilber KS, Anger JT. Abdominal approaches to pelvic prolapse repairs. Curr Opin Urol. 2013;23(4):306–11. doi: 10.1097/MOU.0b013e3283619e36. [DOI] [PubMed] [Google Scholar]
- 4.Mueller ER, Kenton K, Tarnay C, et al. Abdominal Colpopexy: Comparison of Endoscopic Surgical Strategies (ACCESS) Contemp Clin Trials. 2012;33(5):1011–1018. doi: 10.1016/j.cct.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M, O'Sullivan D, Tulikangas PK. A comparison of costs for abdominal, laparoscopic, and robot-assisted sacral colpopexy. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(2):223–8. doi: 10.1007/s00192-008-0744-2. [DOI] [PubMed] [Google Scholar]
- 6.Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175(2):326–33. doi: 10.1016/s0002-9378(96)70142-4. discussion 333-5. [DOI] [PubMed] [Google Scholar]
- 7.Brink CA, Wells TJ, Sampselle CM, Taillie ER, Mayer R. A digital test for pelvic muscle strength in women with urinary incontinence. Nurs Res. 1994;43(6):352–6. [PubMed] [Google Scholar]
- 8.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 9.van Agt HME, Essink-Bot M-L, Krabbe PFM, Bonsel GJ. Test-retest reliability of health state valuations collected with the EuroQol questionnaire. Soc Sci Med. 1994;39(11):1537–1544. doi: 10.1016/0277-9536(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Barber MD, Walters MD, Cundiff GW, PESSRI Trial Group Responsiveness of the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1492–8. doi: 10.1016/j.ajog.2006.01.076. [DOI] [PubMed] [Google Scholar]
- 11.Hunskaar S, Vinsnes A. The quality of life in women with urinary incontinence as measured by the sickness impact profile. J Am Geriatr Soc. 1991;39(4):378–82. doi: 10.1111/j.1532-5415.1991.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 12.Rogers RG, Kammerer-Doak D, Villareal A, Coates K, Qualls C. A new instrument to measure sexual function in women with urinary incontinence or pelvic organ prolapse. Am J Obstet Gynecol. 2001;184(4):552–8. doi: 10.1067/mob.2001.111100. [DOI] [PubMed] [Google Scholar]
- 13.Barber MD, Kenton K, Janz NK, et al. Validation of the activities assessment scale in women undergoing pelvic reconstructive surgery. Female Pelvic Med Reconstr Surg. 2012;18(4):205–210. doi: 10.1097/SPV.0b013e31825e6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollenbeck BK, Dunn RL, Wolf JS, Jr, et al. Development and validation of the convalescence and recovery evaluation (CARE) for measuring quality of life after surgery. Qual Life Res. 2008;17:915–926. doi: 10.1007/s11136-008-9366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber MD, Janz N, Kenton K, et al. Validation of the surgical pain scales in women undergoing pelvic reconstructive surgery. Female Pelvic Med Reconstr Surg. 2012;18(4):198–204. doi: 10.1097/SPV.0b013e31825d65aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogatko A, Babb JS, Wang H, Slifker MJ, Hudes GR. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin Cancer Res. 2004;10(14):4645–51. doi: 10.1158/1078-0432.CCR-03-0535. [DOI] [PubMed] [Google Scholar]
- 17.Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Robotic-assisted and laparoscopic sacrocolpopexy: comparing operative times, costs and outcomes. Female Pelvic Med Reconstr Surg. 2011;17(1):44–9. doi: 10.1097/SPV.0b013e3181fa44cf. [DOI] [PubMed] [Google Scholar]
- 18.Hoyte L, Rabbanifard R, Mezzich J, Bassaly R, Downes K. Cost analysis of open versus robotic-assisted sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2012;18(6):335–9. doi: 10.1097/SPV.0b013e318270ade3. [DOI] [PubMed] [Google Scholar]
- 19.Elliott CS, Hsieh MH, Sokol ER, Comiter CV, Payne CK, Chen B. Robot-assisted versus open sacrocolpopexy: a cost-minimization analysis. J Urol. 2012;187(2):638–43. doi: 10.1016/j.juro.2011.09.160. [DOI] [PubMed] [Google Scholar]
- 20.Wright JD, Burke WM, Wilde ET, et al. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol. 2012;30(8):783–91. doi: 10.1200/JCO.2011.36.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17(4):493–9. doi: 10.1016/j.jmig.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Collinson FJ, Jayne DG, Pigazzi A, et al. An international, multicentre, prospective, randomised, controlled, unblended, parallel-group of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27(2):233–41. doi: 10.1007/s00384-011-1313-6. [DOI] [PubMed] [Google Scholar]
- 23.Breitenstein S, Nocito A, Puhan M, Held U, Weber M, Clavien PA. Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study. Ann Surg. 2008;247(6):987–93. doi: 10.1097/SLA.0b013e318172501f. [DOI] [PubMed] [Google Scholar]
- 24.Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol. 2011;118(5):1005–13. doi: 10.1097/AOG.0b013e318231537c. [DOI] [PubMed] [Google Scholar]