Abstract
Spatial variations in the microstructure of dentin contribute to its mechanical behavior.
Objective
The objective of this investigation was to compare the microstructure and fatigue behavior of dentin from donors of two different countries.
Methods
Caries-free third molars were obtained from dental practices in Colombia, South America and the US to assemble two age-matched samples. The microstructure of the coronal dentin was evaluated at three characteristic depths (i.e. deep, middle and superficial dentin) using scanning electron microscopy and image processing techniques. The mechanical behavior of dentin in these three regions was evaluated by the fatigue crack growth resistance. Cyclic crack growth was achieved in-plane with the dentin tubules and the fatigue crack growth behavior was characterized in terms of the stress intensity threshold and the Paris Law parameters.
Results
There was no difference in the tubule density between the dentin of patients from the two countries. However, there were significant differences (p≤0.05) in the tubule lumen diameters between the two groups in the deep and peripheral regions. In regards to the fatigue resistance, there was a significant increase (p≤0.05) in threshold stress intensity range, and a significant decrease in fatigue crack growth coefficient with increasing distance from the pulp in teeth from the US donors. In contrast, these properties were independent of location for the dentin of teeth from the Colombian donors.
Conclusions
The microstructure of dentin and its mechanical behavior appear to be a function of patient background, which may include environmental factors and/or ethnicity.
Keywords: dentin, fatigue crack growth, fracture, microstructure, strength, tubules
INTRODUCTION
Amongst the three hard tissues occupying human teeth, dentin occupies the majority by both weight and volume. Both coronal and radicular dentin are traversed by a network of tubules (approx. 0.5 to 1.5 µm in diameter) that radiate outward from the pulp cavity to either the dentin enamel junction (DEJ) or cementum [Nanci, 2008]. Regarded as the dentin tubules, each consists of a central lumen that is lined by a collagen-free, hyper-mineralized cuff of peritubular dentin, sometimes called intratubular dentin. Intertubular dentin occupies the interstitial space between the tubules and consists of a collagen fibril matrix reinforced by nanoscale crystals of apatite [Marshal et al., 1997]. Owing to its composition and presence of the tubules, the microstructure of dentin is unique from that of the other hard tissues. Understandably, dentin is often regarded as a hierarchical biological composite [Kinney et al., 2003].
Microscopic evaluations of dentin have been largely focused on characteristics of tubule densities. Overall, the density ranges from roughly 10,000 to over 60,000 tubules /mm^2 [Garberoglio and Brannstrom, 1976; Mjör and Fejerskov, 1979; Pashley, 1989; Mjör and Nordahl, 1996]. There are changes in the number of tubules and their diameter in the tooth crown, which results in spatial variations of the microstructure. These characteristics vary primarily with distance from the pulp, but also depend on the physiology and traumatic history of the tooth. Nevertheless, the tubule density and the tubule diameters are lowest at the DEJ and highest in deep dentin, nearest the pulp chamber [Garberoglio and Brannstrom, 1976]. Pashley [1989] estimated that the tubule lumens occupied approximately 22% of the evaluated cross-sectional area near the pulp, and only about 1% near the DEJ.
According to the large variations in microstructure, the mechanical behavior of dentin within the crown would be expected to be site-specific. Indeed, experimental studies have identified that there are variations in mechanical properties within the tooth crown, including hardness [Pashley et al., 1985, Kinney et al., 1996; Fuentes et al., 2003], ultimate tensile strength [Carvalho et al., 2001, Staninec et al., 2002; Inoue et al., 2003] and shear strength [Watanabe et al., 1996; Konishi et al., 2002]. Pashley et al. [1985] found that the reduction in dentin microhardness from the DEJ towards the pulp was correlated with the increase in tubule density. That reduction in hardness was attributed to the increase in tubule diameter and the reduction in volume of intertubular dentin. Kinney et al., [1996] reported that the spatial variations in macroscopic hardness are also related to intrinsic changes to the intertubular dentin with location and greater hardness near the DEJ. The increase in tubule density with depth is also important to the tensile strength of dentin. Deep coronal dentin is weaker than dentin located near the DEJ, and the differences have been attributed to tubule density [Carvalho et al., 2001, Inoue et al., 2003]. A similar dependence of strength on tubule density has been identified in the root [Giannini et al., 2004].
While the hardness and strength are important measures of mechanical behavior, both being relevant to the success of dental practice, most mechanical forms of tooth failures are expected to occur by fatigue and fracture [Arola et al., 1999; Nalla et al., 2003]. Consistent with the bulk properties, the fatigue and fracture resistance of dentin are strongly influenced by spatial variations in the microstructure. Ivancik et al., [2011] identified that there is a significant decrease in the fatigue crack growth resistance of coronal dentin with increasing distance from the DEJ. Cracks in deep dentin (close to the pulp) begin to undergo cyclic extension at stresses nearly 40% lower than that in tissue nearer to the DEJ. According to microstructural analysis, the average fatigue crack growth rates increased significantly with increasing tubule density. The fracture toughness of dentin is a function of location as well, with an increase in fracture toughness of nearly 50% from deep dentin to the outer regions [Ivancik et al., 2013]. There is substantial clinical significance to the aforementioned findings, namely that restorations extended into deep dentin are much more likely to facilitate tooth fracture. Flaws resulting from cavity preparations in deep dentin will undergo more rapid cyclic crack growth, and will reach a critical length much sooner than that in the peripheral regions.
Although the spatial variations in microstructure of dentin are well-recognized, to the authors’ knowledge no study has questioned whether the microstructural variations are equivalent amongst all individuals, or if they are dependent on the patient background, e.g. living environment or ethnicity. A comparison of the fatigue properties of dentin obtained from senior residents in the US and China reported a significant difference in fatigue crack growth resistance between the two groups [Arola et al., 2010]. However, that study did not perform a thorough comparison of the microstructures. Environmental and/or ethnic factors could be important to the microstructure of dentin, thereby resulting in differences between patients of disparate groups. In recognition of past studies, differences in the microstructure would reflect on the mechanical behavior of dentin. Thus, the objective of this investigation was to compare the microstructure and corresponding fatigue crack growth resistance of dentin from age-matched residents of North and South America. The null hypothesis of the investigation is that there are no differences in the microstructure of dentin or its fatigue resistance from these two populations.
MATERIALS AND METHODS
Non-carious third molars were obtained from donors in two locations, namely patients visiting private practices in the state of Maryland and patients visiting practices in the city of Medellín, which is located in the Department of Antioquia, Colombia. These two locations were chosen for the collaborative relationship that has been established between EAFIT University and CES University of Medellín and the University of Maryland. Details regarding the potential differences between donor groups in these two locations will be discussed later. In both countries, the teeth became available as a result of routine extractions necessary for maintaining oral health of the patients. All collected teeth were obtained from donors between the ages of 18 and 35 years old, an age span defined by earlier studies to exhibit consistent fatigue and fracture behavior [Arola and Reprogel, 2005; Nazari et al., 2009; Ivancik et al., 2012]. Those teeth from donors in the US were obtained in accordance with an approved protocol issued by the Institutional Review Board of the University of Maryland County (Approval Y04DA23151). In Colombia, the teeth were obtained with written consent and followed the protocols required by both the Cooperative University of Colombia (UCC) Dental Clinic and EAFIT University. In both locations the teeth were placed in Hanks Balanced Salt Solution (HBSS) immediately after extraction along with record of donor age and gender.
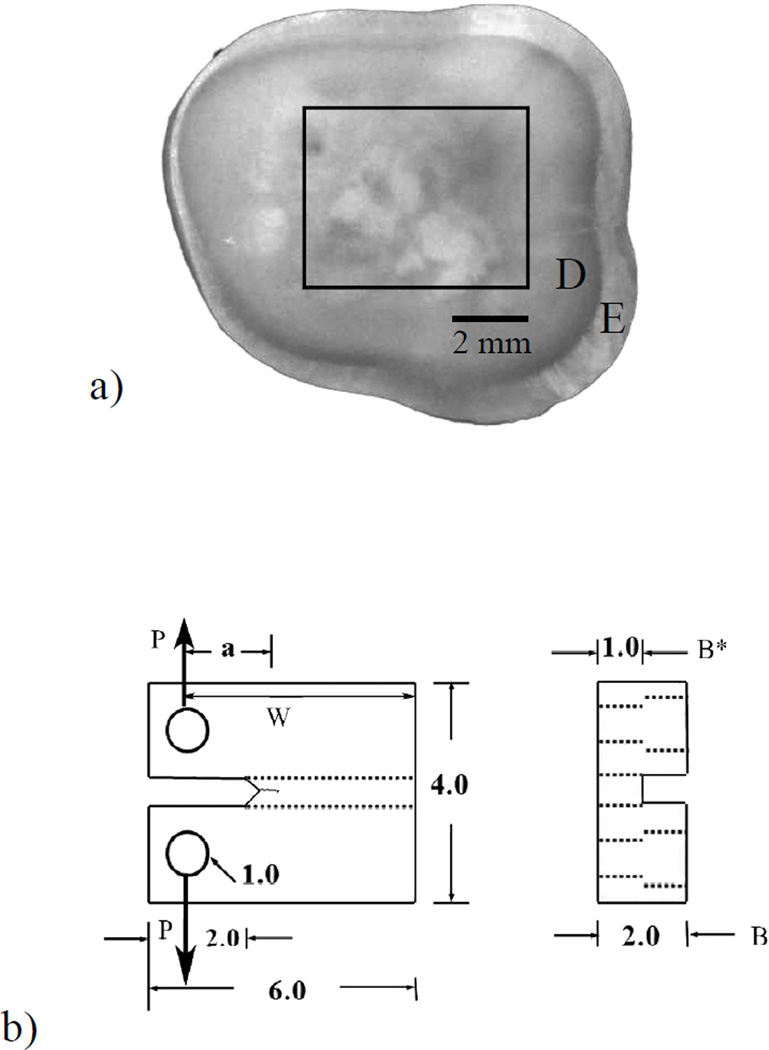
Evaluations of the microstructure and fatigue resistance for both groups of teeth were conducted within the US. Within one month of extraction, the teeth were sectioned using a numerical controlled slicer/grinder (Chevalier, Model SMART-H81811, Taiwan) under water-based coolant using diamond abrasive slicing wheels. Serial sections were made perpendicular to the tooth’s axis to obtain a single 2 mm thick section (Figure 1(a). Secondary sectioning was then performed to obtain Compact Tension (CT) specimens as shown in Figure 1(b). The specimens were obtained as a function of relative distance from the DEJ in three general regions, including deep dentin (nearest the pulp), middle dentin (tissue located centrally between the pulp and DEJ) and superficial dentin (the peripheral region closest to the DEJ). It is important to note that only one specimen was obtained from each tooth. In some of the largest teeth more than one sample could be prepared from the crown tissue (e.g. from the deep and superficial dentin). But this is rare, and seldom possible with the teeth from Colombia due to the smaller size. Thus, to minimize the chances for conflicting degrees of independence between the two sample groups, only one sample was prepared from each tooth. Based on the location and manner of sectioning, the dentin tubules were oriented in-plane with the fatigue crack surface, and perpendicular to the direction of crack extension. A total of N=43 teeth were evaluated from donors of the two countries. In terms of the three regions of evaluation, the investigation consisted of 14 specimens of deep dentin (US n=10; Colombia n=4), 16 specimens of middle dentin (U.S. n=12; Colombia n=4) and 13 specimens from the superficial region (US n=9; Colombia n=4).
Figure 1.
Preparation of a compact tension (CT) specimen from the coronal dentin of a 3rd molar. (a) view of an axial section from a tooth crown and potential location of a specimen. E and D indicate the enamel and dentin, respectively; (b) final geometry and dimensions of the CT specimen. Loading (P) is applied from pins placed within the two holes.
Cyclic loading of the CT specimens was performed using a BOSE ElectroForce 3200 (Eden Prairie, MN, USA) universal testing system using routine methods developed and applied in previous studies [Bajaj et al., 2006; Ivancik et al., 2011]. Briefly, the specimens were subjected to cyclic loading at a frequency of 5 Hz and under load control displacement. All testing was conducted while the specimens were immersed in HBSS maintained at room temperature (22°C). The loads were applied in opening mode (Mode I) in which two pins were placed within the opposing holes of the CT specimen (Figure 1(b). Crack initiation was achieved using a stress ratio (R) of 0.5, which defines the ratio of minimum and maximum load. Once the crack was initiatied, cyclic crack growth was conducted using R = 0.1. Thereafter, the specimens was subjected to increments of cyclic loading, followed by an assessment of the crack extension. Crack length measurements were achieved using a digital microscope (Navitar IEEE 1394) at a magnification of 60X. The number of cycles between measurements (ΔN) was chosen according to the observed crack growth rate and typically ranged between 5 and 20 kcycles; the average increment of extension was between 60 to 120 µm. The incremental crack growth rates (da/dN) were quantified by dividing the measured incremental crack extension (Δa) by the increment of loading cycles (ΔN). These methods are consistent with those used in previous studies concerning fatigue crack growth behavior of dentin [Bajaj et al., 2006; Ivancik et al., 2011; Ivancik et al., 2012].
Most of the fatigue crack growth responses exhibited three characteristic regimes of fatigue behavior including initiation, steady-state incremental growth, and the tertiary regime of unsteady crack extension to fracture. The incremental fatigue crack growth rate (da/dN) within the region of steady state (Region II) response was quantified using the Paris Law [Paris et al., 1961] according to
| (1) |
where ΔK is the stress intensity range, and the quantities C and m are the fatigue crack growth coefficient and exponent, respectively. The stress intensity range (ΔK) is determined from the difference in stress intensity at the minimum and maximum loads [ASTM, 2013]. Due to the unique specimen size, the stress intensity resulting from cyclic loading was estimated according to the work of Bajaj et al., [2006]. Using the incremental crack length measurements and the corresponding stress intensity, the fatigue crack growth rate (da/dN) were plotted in terms of ΔK to estimate the quantities m and C for each specimen. In addition, the apparent stress intensity threshold range (ΔKth) was estimated from the intercept of the responses at an apparent fatigue crack growth rate of 1×10^−7 mm/cycle. Thus, results obtained from each specimen include measures of the ΔKth and the two Paris Law parameters (C and m). The cumulative responses for each parameter corresponding to the two different groups of donors were defined in terms of the average response from individual specimens of each region. A comparison of the fatigue crack growth parameters for specimens obtained from each donor group was conducted using an Analysis of Variance (ANOVA) with significant differences identified by p≤0.05. A comparison of the responses from amongst the three depths was performed using Tukey’s Honestly Significantly Different (HSD) test.
After completion of the fatigue crack growth experiments, the CT specimens where dehydrated in air for a day, and then sputter-coated (Model LLC Desk II, Denton vacuum, Moorestown, NJ, USA) with gold-palladium alloy. The fracture surfaces and the overall microstructure were evaluated using scanning electron microscopy (SEM; JEOL JSM 5600, JEOL Inc., Peabody, MA) under secondary electron imaging and/or back scatter emission modes. The microstructural characteristics of the dentin from the two donor groups were examined quantitatively to identify if there are any fundamental differences that could contribute to the fatigue crack growth behavior. To facilitate clear identification of the lumen dimensions and peritubular cuff, the samples were polished briefly using diamond particle suspensions (Buehler) of sizes 9 µm, 3 µm, and 0.04 µm. All polishing was performed by hand on a standard cloth wheel mounted on top of a granite surface plate. The polishing was performed without application of pressure. A commercial image analysis software (Image J 1.42, National Institute of Mental Health, Bethesda, Maryland, USA) was used to count the number of lumens per unit area, and in quantifying the average lumen dimensions in each of the three regions of evaluation. Significant differences in the microstructure were identified using an ANOVA with p≤0.05
RESULTS
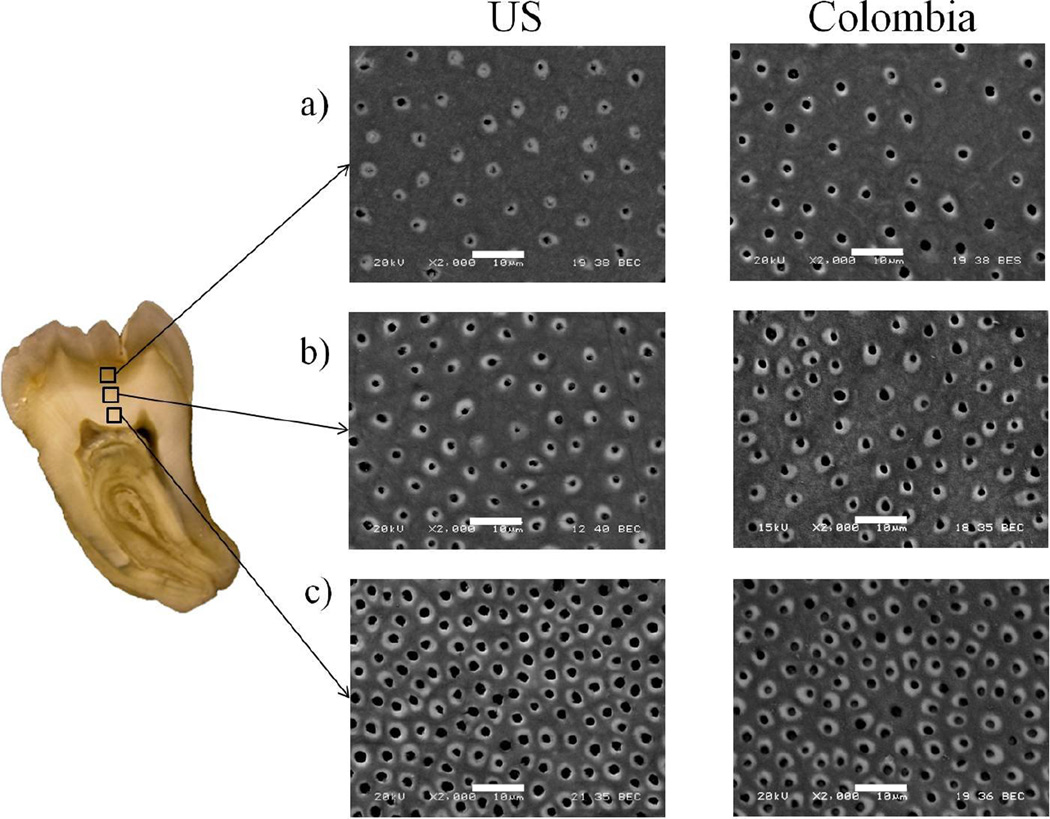
A comparison of the microstructure of dentin specimens representing the two groups of donors is presented in Figure 2. Note that pairs of micrographs are shown for superficial or peripheral dentin (Figure 2(a), central or middle dentin (Figure 2(b) and deep dentin (Figure 2(c), with one image from each of the two donor groups. As evident from these images, there is a reduction in the tubule density with increasing distance from the pulp for both donor groups. Overall, the lumen density ranged from approximately 10,000 to 60,000 lumens per mm2 and 15,000 to 43,000 lumens per mm2 for the teeth of donors from the US and Colombia, respectively.
Figure 2.
SEM micrographs of the dentin microstructure for specimens of selected US and Colombia donors. a) Peripheral dentin; b) Central dentin; c) Deep dentin. The scale markers in each micrograph represent 10 µm. Note that both patient groups were found to have comparable lumen densities for the three evaluated regions. There is an increase in lumen diameter with depth evident in the dentin from US donors, whereas the average lumen diameter appears nearly constant for the Colombian dentin in the three regions of the crown.
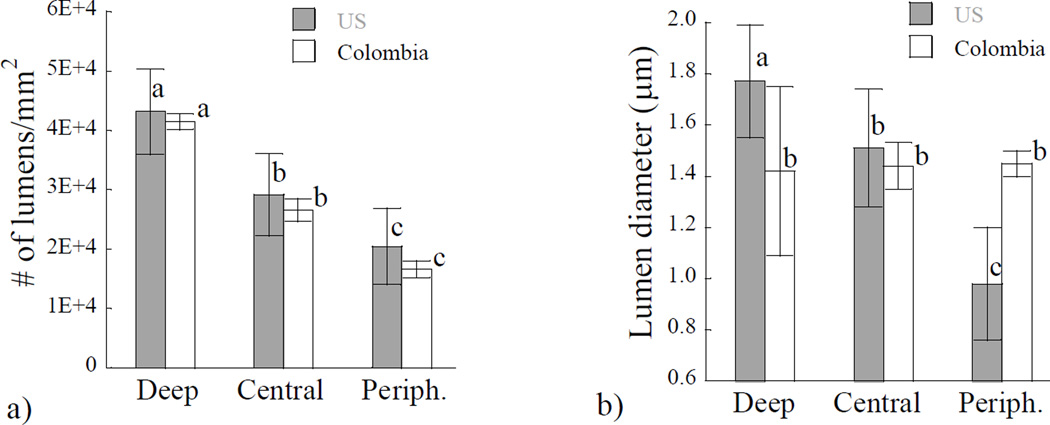
A quantitative comparison of the microstructural characteristics of dentin from the two donor groups is shown in Figure 3. In Figure 3(a) the average lumen density is presented within the three separate regions of evaluation. There was a significant reduction in the lumen density with depth (p≤0.05), for both of the donor groups. However, there was no significant difference (p>0.05) in the tubule density between the two donor groups within any of the three regions. A comparison of the average lumen diameters within the three regions of evaluation and for the two donor groups is shown in Figure 3(b). For the donor group of the US, there was a significant decrease in lumen diameter from the peripheral region to the deep dentin, with average lumen diameters in these regions of approximately 1.0 µm and 1.8 µm, respectively. However, for the teeth from Colombian donors there was no significant change in the lumen diameter with depth. The average diameter for this donor gropu considering all three regions was roughly 1.4 ± 0.2 µm; the largest varation in this dimension was noted in the deep dentin.
Figure 3.
A comparison of the microstructure as a function of location in the coronal dentin from donor teeth of the US and Colombia. a) lumen density; b) lumen diameter. Columns with different letters are significantly different (p≤0.05).
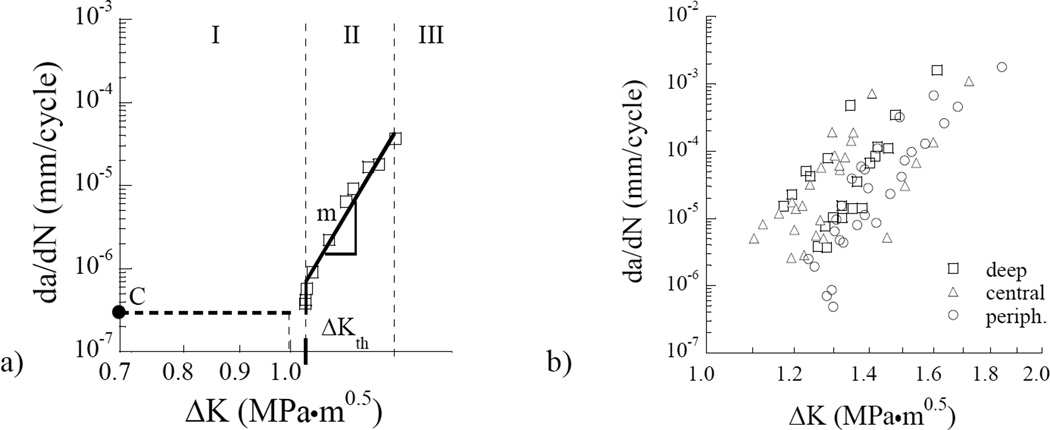
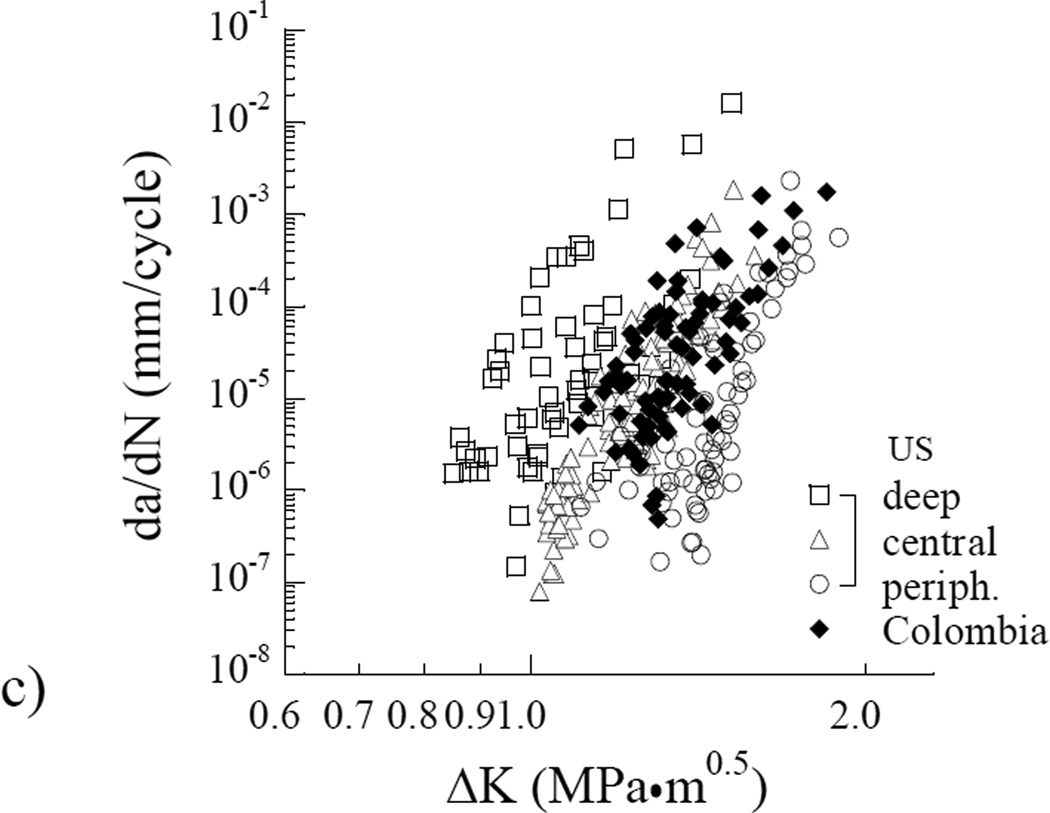
A typical fatigue crack growth history from the dentin of a single donor is shown in Figure 4(a). This responses is annoted to highlight the three regions of responses, as well as the three measures of cyclic extension obtained for each specimen. The cumulative fatigue crack growth responses for all specimens obtained from the Colombian donors are shown as a function of the stress intensity range in Figure 4(b). A comparison of these responses with those obtained from the dentin of donors in the US is shown in Figure 4(c). Apparent from this comparison, the overall fatigue crack growth behavior of the two donor groups is consistent. However, the responses obtained from the donors of Colombia appear to exhibit a much smaller range of fatigue resistance, and show less dependence on location in which the tissue was obtained.
Figure 4.
Fatigue crack growth (FCG) resistance of coronal dentin. (a) A typical fatigue crack growth response distinguishing all three regions of cyclic extension; (b) A comparison of the resistance to cyclic extension for coronal dentin obtained from the Colombian donors. The responses are divided into specimens obtained from the inner, central and peripheral regions. c) Comparison of the fatigue crack growth responses of specimens obtained from both donor groups (US and Colombia). Results from (b) for the Colombian donors are not denoted by region in (c). Note the consistency in results for the Colombian responses in comparison to those for the US donor group.
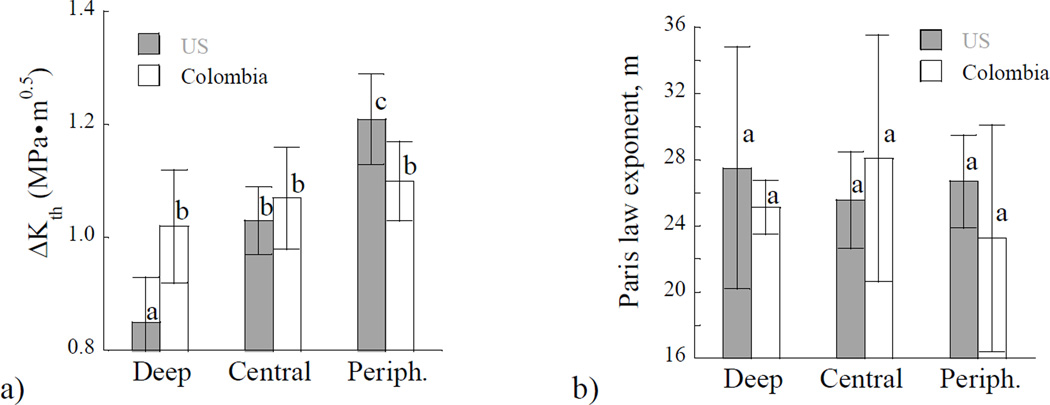
A quantitative assessment of the fatigue crack growth results for the individual specimens provided the stress intensity threshold range, and the two Paris Law parameters describing the steady-state growth behavior. The average values of these parameters were estimated as a function of dentin depth and are shown in Figure 5. The importance of location on the stress intensity threshold range for the two donor groups is shown in Figure 5(a). In comparing results from the two donor groups, there were differences in the fatigue crack growth initiation resistance including the magnitude and spatial dependence. For dentin obtained from US donors the stress intensity threshold range from the inner (0.8±0.1 MPa•m^0.5) dentin was significantly lower (p≤0.0001) than that from the peripheral region (1.2±0.1 MPa•m^0.5). However, for dentin of Colombian donors, there was no significant difference in the average stress intensity threshold range obtained for the three regions; the overall average value for this group was 1.1±0.1 MPa•m^0.5. In comparing results obtained by region, there were significant differences (p≤0.05) between the two groups in the deep and peripheral dentin.
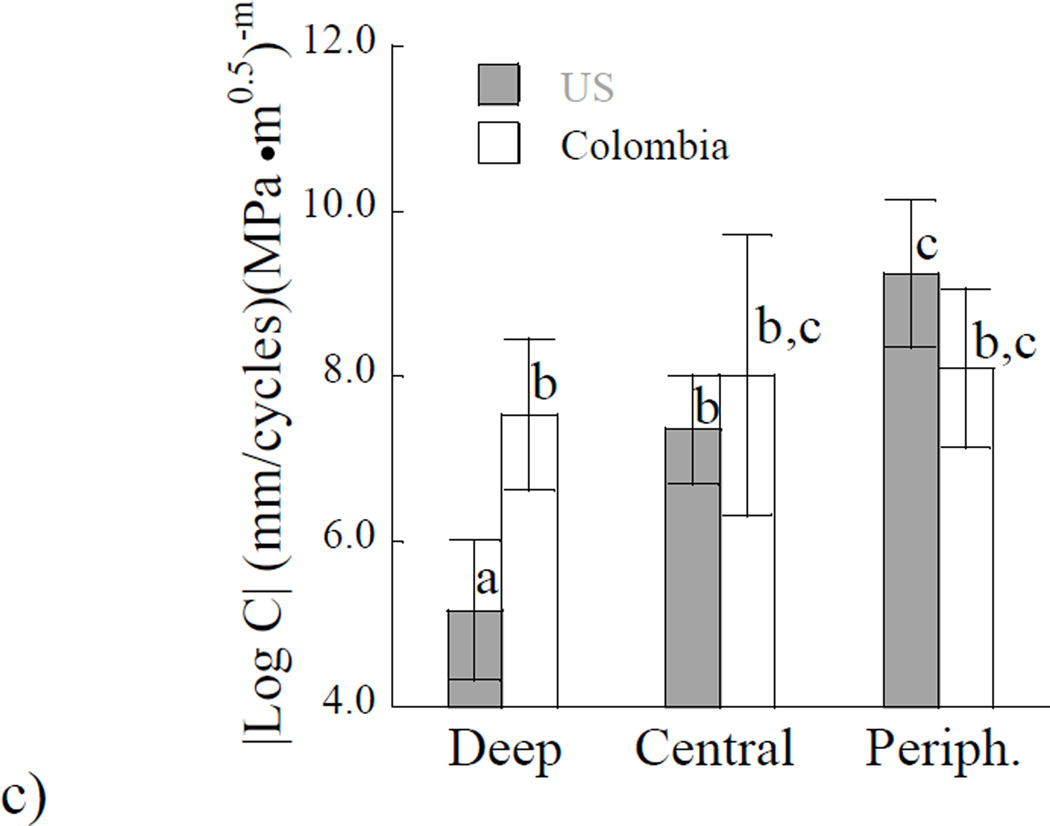
Figure 5.
A comparison of the Paris Law parameters for the two donor groups and differences with location. a) Stress intensity threshold; b) Paris law exponent m. c) Log of the Paris law coefficient C. Columns with different letters are significantly different (p≤0.05).
Results obtained for the steady-state region of fatigue crack growth are presented in Figures 5(b) and 5(c). Specifically, the importance of location on the average fatigue crack growth exponents for the two groups of donors is shown in Figure 5(b). There was no significant difference (p>0.05) in the exponents with respect to location or donor group. The average Paris Law exponent for dentin of the US and Colombian donors was approximately 27±8 and 25±9, respectively. The fatigue crack growth coefficients obtained from specimens at the three depths of evaluation are shown in Figure 5(c). For clarity of presentation, the average values at each depth are presented in terms of absolute value of the log base 10. In evaluation of results for the US donor group, there was a significant increase (p≤0.01) in the fatigue crack growth coefficient with depth, indicating that the greatest incremental fatigue crack growth rate occurred in deep dentin. However, for the donor group of Colombia, there was no significant difference in the fatigue crack growth coefficient amongst the three locations. In comparing results from the two donor groups by region, there was a significant difference (p≤0.05) in the fatigue crack growth coefficients of the deep dentin only.
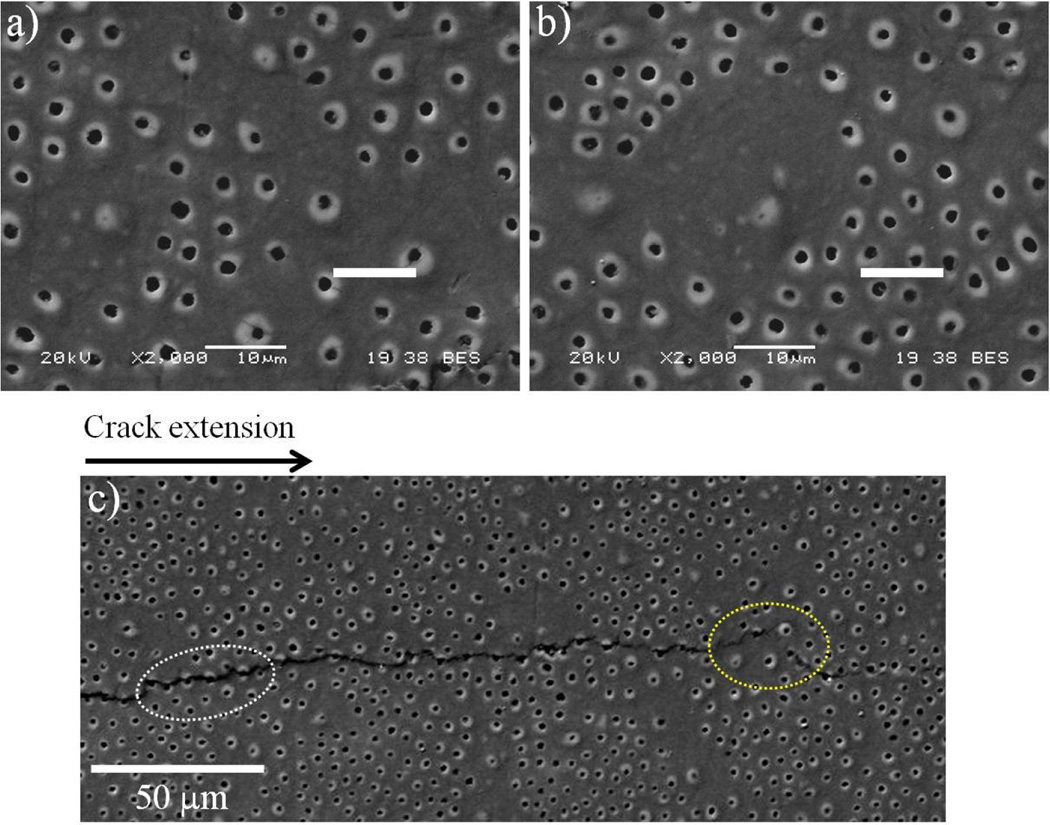
During analysis of the fatigue crack growth behavior, it was noted that some aspects of cyclic extension were not directly represented by the quantitative responses. For example, some of the specimens exhibited locations with an irregular tubule distribution, or locations without lumens. These observations were most common in dentin from the Colombian donors. While regions with this characteristic were not included in the quantitative estimates of tubule density and lumen diameter, they were important to the fatigue crack growth responses. Selected specimens with this characteristic are shown in Figure 6(a) and Figure 6(b). A crack path corresponding to cyclic crack extension within a dentin specimen obtained from a Colombian donor is shown in Figure 6(c); the direction of crack extension is from left to right. In regions with sparse or irregular lumens, the crack growth process was temporarily interrupted. In these locations the crack would undergo a local change in orientation that followed a more favorable path consisting of greater lumen density, as evident in Figure 6(c). Highlighted in this micrograph are the locations where the crack extended uniformly, in regions of evenly distributed lumens (encircled in white), and a region of interruption (encircled in yellow) where the crack extended into an area of sparse tubules (yellow). In regions of low lumen density, it was noted that the crack underwent a decrease in the growth rate or even temporary crack arrest.
Figure 6.
Micrographs detailing the microstructure of dentin from the teeth of Colombian donors. a) and b) show examples of non-uniform lumen distribution in two different specimens. The scale markers represent 10 µm. c) Crack extension from left to right in the central region obtained from a 21 year old female donor. Note the path of crack extension (enclosed in white) extends from “lumen to lumen” and that a change in path occurs in the region that exhibits a nonuniform tubule pattern (enclosed in yellow).
Based on observations of cyclic crack growth from the surface used in viewing and measuring the crack length, cyclic extension occurred through a connection of fracture events that developed in adjacent lumens. Generally the crack would extend from lumen to neighboring lumen along a path of large tubule density, where the distance between lumens was shortest. The extension to adjacent lumens did not occur every cycle, but rather after tens of cycles or more. There was consistency in this behavior for both donor groups, suggesting that the toughening mechanisms contributing to the crack growth resistance were similar. These included crack bridging posed by unbroken ligaments of tissue and collagen fibrils of matrix that were active behind the crack tip, microcracking of the peritubular cuffs within the frontal zone of the crack-tip, and also localized crack curving. The latter mechanism was found to be highly influenced by the tubule density, and was most commonly noted in regions of irregular tubule distribution, as shown in Figure 6(c).
DISCUSSION
In this investigation, the microstructure and fatigue crack growth resistance of dentin from donor teeth of patients living in selected countries of North and South America were evaluated. To the author’s knowledge, the present investigation is the first to compare the microstructure of dentin from patients of different countries, and to consider this information in an exploration of the mechanical behavior relevant to the success of restorative dentistry.
The three most common forms of restored tooth failure are recurrent caries, deterioration of the margins and tooth fracture. Approximately one-half of all dental restorations fail within 10 years due to secondary caries and fracture [Mjor and Toffeneti, 2000; Sarret, 2005; Ferracane 2011]. Cracking and tooth fractures are an impediment to lifelong oral health [e.g. Geurtsen et al., 2003; Roh and Lee, 2006]. The incidence of tooth fractures is not only a concern in the US, but also in the countries of South America [e.g. Naranjo, 2007; Ramirez, 2008]. As tooth fracture is generally facilitated by cyclic crack extension of existing flaws in dentin [Arola et al., 1999; Nalla et al., 2003], the fatigue crack growth resistance is of substantial relevance. Furthermore, teeth with cracks are difficult or even impossible to repair – they are often extracted! The best approach for preventing tooth fracture today is the development of clinical practices guided by knowledge on the fracture resistance of teeth. Hence, this study was motivated in recognition of the importance of tooth fracture to lifelong oral health within the two countries.
For both donor groups, the measures of microstructure were within the ranges previously reported for tubule density [e.g. Garberoglio and Brannstrom, 1976; Pashley, 1989] and tubule diameter [e.g. Schilke et al., 2000; Coutinjo et al., 2007]. Surprisingly, although the average dentin tubule density was consistent between the two donor groups, there were significant differences in the tubule diameters. Thus, the null hypothesis must be rejected. In the coronal dentin of teeth from US donors, the average lumen diameter was found to increase with depth, whereas the lumen diameter was essentially a constant value throughout the crown in Colombian teeth. Similar results have been reported in a microstructural analysis of dentin in Brazilian teeth [Coutinho et al., 2007] indicating a constant lumen diameter over the crown depth. The overall average lumen diameter in the teeth of Colombian donors (1.4 ± 0.2 µm) was equivalent to that in the middle region of teeth obtained from US donors. The largest difference in microstructure between the two groups was identified in the peripheral dentin (Figure 3(b), where the average lumen diameter of tissue from the Colombian donors was over 40% greater than that from the dentin of US donors.
Trends in the dentin microstructure were reflected in the fatigue crack growth resistance of the two groups of donor teeth. With respect to spatial variations in properties, it was found that there was a significant reduction in the fatigue crack growth resistance with depth in dentin from US donor teeth only. The spatial variations agree with results of previous studies reporting on the fatigue crack growth properties and fracture toughness of dentin [Ivancik et al., 2011, Ivancik and Arola, 2013]. These prior studies were conducted on tissue obtained from teeth of US donors. In both loading formats, deep dentin showed the lowest resistance to crack extension. However, in the tissue from Colombian donors there was no significant changes in either the initiation behavior or the steady-state fatigue crack growth behavior with location. The ΔKth and Paris Law parameters (m and C) remained constant in this group as evident in Figure 5. The average values for ΔKth and C of the three regions were 1.1 MPa•m0.5 and 1.78E-08 (mm/cycle)(MPa•m0.5)−m, respectively. These values were also consistent with the averages for central dentin obtained from US donors (ΔKth = 1.03 MPa•m0.5, C = 4.41E- 08 (mm/cycle)(MPa•m0.5)−m). When the stress intensity threshold range of the two groups are compared by region, there were significant differences (p≤0.05) between the two groups for both the deep and peripheral dentin. Neither donor group showed spatial variations in the fatigue crack growth exponent (Figure 5(b). However, there was a significant difference in C of the deep dentin between the two groups, which manifested as a larger incremental crack growth rate in dentin of the US donor teeth.
On factor to consider in comparing the fatigue crack growth parameters and average crack growth rates of the two donor groups is the difference between the experimental conditions and those of actual mastication. The experiments were conducted within a hydration bath, which maintained the moisture content of the dentin samples. Nevertheless, the experiments were conducted at room temperature rather than 37°C. Thus, the difference in temperature does not appear to be an important factor [Yahyazadehfar et al., 2014]. Another concern is loading frequency. According to Braem et al. [1994] the upper range of chewing frequency is ~2 Hz, while in this study a frequency of 5 Hz was used. The higher frequency was chosen to maintain consistency with earlier studies on fatigue crack growth in human dentin [Bajaj et al., 2006; Ivancik et al., 2011; 2012] and to balance the concerns associated with clinical relevance and the time required to achieve meaningful data. A single experiment at 5 Hz typically extends over a period of one week, and a reduction of frequency results in substantial changes in time required for the investigation. Kruzic et al. [2005] showed that higher frequencies can promote slower crack growth rates within dentin. While these studies were conducted on elephant tusk dentin, the findings suggest that the measured growth rates obtained at 5 Hz may be slightly lower than those resulting from mastication.
Results from the experiments showed that the fatigue crack growth responses of dentin from the Colombian donors did not depend on location. Spatial variations were only found in dentin of the US donors. Reflecting on the microstructural measurements, the only notable difference in microstructure between the two groups is the lumen diameter. That would suggest that the lumen diameter is the primary factor regulating the spatial aspects of fatigue crack growth resistance, and that it decreases with increasing lumen diameter. Indeed, the mechanism of cyclic crack extension in this orientation is from lumen to lumen, and the larger lumen size results in an elevated average stress due to the stress concentration. The relatively constant lumen diameter of dentin from the Colombian teeth (Figure 3(b) appears to homogenize the fatigue responses. While the influence of microstructure on fatigue crack growth resistance is interesting, there are clear implications to the propensity for restored tooth fracture. For shallow restorations extending only into peripheral dentin, there is greater probability for fatigue crack growth to cause tooth fractures in the teeth of Colombian patients. However, for restorations extending into the deep dentin, there is a greater probability of fatigue crack growth and consequent tooth fracture in the patients of the US.
The microstructural evaluation concentrated on the dimensional characteristics of the dentin. That has been the most common method to characterize the variations in microstructure of dentin over the tooth [e.g. Garberoglio and Brannstrom, 1976; Mjör and Fejerskov, 1979; Pashley, 1989; Mjör and Nordahl, 1996; Schilke et al., 2000]. But there are other aspects of the tissue that exhibit spatial variations as well, including the relative mineral and organic content. For example, Tesch et al [2001] evaluated the spatial variations in intertubular dentin of the crown using a host of quantitative approaches. From a comparison of correlations, it was identified that the relative mineral content and the thickness of the mineral crystals were the best predictors of nanohardness and elastic modulus of intertubular dentin. Ryou et al. [2011] evaluated the strength, as well as the strain and energy to fracture of coronal dentin and found that the trends in mechanical behavior were consistent with the mineral/collagen ratios. Deep dentin exhibited the highest mineral/collagen ratios and highest “brittleness” as indicated by the low strength and strain to fracture. Consistent with the findings of Staninec et al [2002], deep dentin also exhibited a larger size distribution of contributing intrinsic flaws. Therefore, in addition to differences in lumen dimensions, there may be aspects of the relative mineral and organic content of the donor groups that contributed to the fatigue resistance. Future studies in this area should consider adopting additional methods of characterization for analyzing the chemical composition and its importance to the spatial variations in fatigue resistance.
The identification of differences in the microstructure raises questions pertaining to the principal cause. Environmental influences, nutrition and oral health could be contributing factors based on their influence to tooth formation and development [Nikiforuk, 1970; Shaw, 1970]. The environmental conditions of Medellín parallel those of US cities, with air quality three times better than the values suggested by the World Health Organization [Alcaldia de Medellín, 2011]. The participating clinics serve an area of Medellín supported by public water, which acheives potable standards, chemistry and quality equivalent to those of the western world [Mejía, 2011]. Furthermore, the nutritional status of those in Medellin is equivalent to that in the US [FAO, 2001] with average adult intake exceeding 2000 cal/day. The average Colombian diet consists of more maize and beans than in the US, but the seven major food groups are represented. Of relevance, similar results in the lumen diameter were reported for the dentin of third molars from donors in Brazil [Coutinho et al., 2007]. Brazil borders Colombia and the general diet and living environment of people in these two countries are very similar. Therefore, disparities in living environment and nutrition do not appear to be contributing factors to the difference in microstructure between the two donor groups.
Other potentially contibuting factors may include difference in the diseases and dental care between the donor groups. According to the statistics reported by the World Health Organziation (WHO, 2010) the 4 primary noncommunicable diseases in Colombia are cardiovascular disease (28%), cancer (17%), respiratory disease (6%) and diabetes (3%). No connection has been made between these diseases and the microstructure or mechanical properties of dentin. The prevalence of diabetes is increasing in Colombia [Aschner, 2002], but it is far lower than that in the US. In regards to oral health, the primary dental diseases are caries, periodontitis and dental fluorosis [ENSAB, 1999]. The prevalence of dental fluorosis in children of Medellin (8%) [Ramirez-Puerta et al., 2009] is higher than in the USA (3%) [Beltran-Aguilar et al., 2005]. However, dental fluorosis is easy to identify and the teeth were screened at receipt to exclude those with potential evidence of fluorosis. Consequently, there does not appear to be contributions from disease or oral health factors to the findings.
There could be differences in ethnic background. In both countries the teeth were collected with record of patient age and gender only, and without identification of patient ethnic background. For the US donor group the collected teeth are considered of “mixed” ethnic background. Considering the demographics of Maryland, the population of donors consisted of roughly 54% white, 30% black, 9% Hispanic and 6% Asian. The Colombian population overall is primarily of European origin (~86%), Amerind (American Indian, ~3.4% ) and Afrocolombians (~10.6%) [DANE, 2007]. According to genetic studies (Carvajal-Carmona et al., 2000; 2003), the demography of Medellín is ~94% European origin (of Spain), ~5% African and ~1% Amerind. Those in the European category are regarded as white or “mestizos” denoting a mixture of European and indigenous Indian background. Furthermore, the participating clinics are located in the El Poblado district of Medellín (strata between 3 and 5), a center of commerce largely populated by persons of European descent (i.e. not African or AmerInd). Thus, it is not clear if the findings are related to differences in ethnicity of the two groups, but it is a relevant consideration. A report issued by the United States Census Bureau [2006] recently provided an evaluation of the Hispanic population in the US. The report details that in 1970 the Hispanic population was comprised of nearly 10 million individuals (approx. 5% of the total US population). Today that number is reaching 45 million (14%) and is expected to surpass 75 million (20%) by the year 2030. Between 2000 and 2006 the growth rate in Hispanics (24.3%) was more than 3 times the growth rate of the total population. Hispanics are the fastest growing minority in the United States. If the mechanical behavior between the two donor groups are related to ethnicity, then the substantial rise in the number of Hispanics in the US could have important implications to the field of dentistry.
Results of the present investigation identified that the structure and fatigue resistance of dentin from the teeth of US and Colombian donor groups exhibit significant differences. Similar differences could exist between other donor groups. Previous studies have compared the structure and/or physical aspects of bovine dentin to human dentin, largely to address whether bovine dentin is an adequate substitute for human tissue [Tagami et al., 1989; Schilke et al., 2000; Camargo et al., 2008; Dutra-Correa et al., 2007; Wegehaupt et al., 2008; Lopes et al., 2009]. Based on the present findings, there may be equal or greater merit to the field of dentistry in exploring the differences between patient groups than animal species. If those studies are conducted, one factor to consider is the impact of aging. There are changes in the microstructure of dentin with aging that result in a reduction of the fatigue crack growth resistance [Bajaj et al., 2006; Ivancik et al., 2012] and fracture toughness [Koester et al., 2008; Nazari et al., 2009]. An earlier comparison of the fatigue crack growth properties of tissue from donors of China and the US reported significant differences [Arola et al., 2010]. If the effects of aging and/or its rate of progression is not consistent amongst patient groups, that will add another dimension to the complexity of restorative dentistry in the future. If one accepts that premise that type II diabetes leads to “accelerated aging”, then tooth dentin of US residents may be physiologically older than aged-matched controls from countries with far less type II diabetes.
CONCLUSIONS
On the basis of the results obtained, the following conclusions may be drawn:
There was no significant difference in the tubule density of coronal dentin from the two donor groups. The overall tubule density ranged from approximately 10,000 to 60,000 tubules/mm^2. There was a significant increase in the tubule density from the dentin enamel junction to the pulp, in teeth from both the US and Colombian donor groups.
In evaluation of the lumen diameters, there was a significant decrease in the lumen diameter with increasing distance from the pulp in dentin from the US donors. However, the average lumen diameter of dentin from the Colombian donors did not change significantly with depth; the average diameter was 1.4 ± 0.2 µm. The tubule lumens in the peripheral dentin of the US donors were significantly smaller than those in the corresponding location of the Colombian donors.
There were significant differences in the fatigue crack growth resistance between the two donor groups. For the teeth obtained from the US donors, the stress intensity threshold range from deep (0.8±0.1MPa•m0.5) dentin was significantly lower (p≤0.0001) than that from the peripheral region (1.2±0.1 MPa•m0.5). However, for dentin from the Colombian donors there was no significant difference in the stress intensity threshold range between the three regions of investigation. The average value was 1.1±0.1 MPa•m0.5
There were no spatial variations in the Paris Law exponents for either donor group. However, in evaluation of the fatigue crack growth coefficient, there was a significant increase in the coefficient with increasing depth for the donor group of the US. In contrast, there was no significant spatial variation in that parameter for the dentin of the Colombian donor group. These spatial variations in the US donor group resulted in a decrease in the fatigue crack growth resistance with distance from the DEJ.
Highlights.
-
-
We evaluate the microstructure of dentin from donor teeth obtained from patients of the US and Colombia.
-
-
We evaluate the fatigue crack growth resistance of dentin from these donor teeth and the spatial variations.
-
-
There are significant differences in the microstructure of coronal dentin by location
-
-
There are significant differences in the microstructure between the dentin of donor teeth from the US and Colombia
-
-
There are significant differences in the fatigue crack growth resistance of the peripheral dentin between the two groups.
ACKNOWLEDGEMENTS
This study was supported by grant NIH R01 DE016904 (PI. D. Arola) from the National Institute of Dental and Craniofacial Research.
Support for the following investigation was provided by the National Institutes of Dental and Craniofacial Research (DE016904).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alcaldia de Medellín. Analisis de la evolucion de la calidad de vida en Medellín, 2008-2011. Medellin, Colombia: Medellín Como Vamos; 2011. (The State of Medellín “Analysis of the evolution of quality of life in Medellin, 2008-2011”. [Google Scholar]
- Arola D, Huang MP, Sultan MB. The failure of amalgam restorations due to cyclic fatigue crack growth. J Mat Sci: Mater Med. 1999;10(6):319–327. doi: 10.1023/a:1026435821960. [DOI] [PubMed] [Google Scholar]
- Arola D, Reprogel R. Effects of aging on the mechanical behavior of human dentin. Biomaterials. 2005;26(18):4051–4061. doi: 10.1016/j.biomaterials.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Arola D, Bajaj D, Ivancik J, Majd H, Zhang D. Fatigue of Biomaterials: Hard Tissues. Int J Fat. 2010;32(9):1400–1412. doi: 10.1016/j.ijfatigue.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner P. Diabetes trends in Latin America. Diabetes Metabolism Research Reviews. 2002;18(3):S27–S31. doi: 10.1002/dmrr.280. [DOI] [PubMed] [Google Scholar]
- ASTM E647-13a, Standard Test Method for Measurement of Fatigue Crack Growth Rates. Annual Book of ASTM Standards, American Society for Testing and Materials, West Conshohocken, PA. 2013;03.01:1–50. [Google Scholar]
- Bajaj D, Nazari A, Sundaram N, Arola D. Aging, dehydration and fatigue crack growth in human dentin. Biomaterials. 2006;27(11):2507–2517. doi: 10.1016/j.biomaterials.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Beltrán-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, Hyman J, Jaramillo F, Kingman A, Nowjack-Raymer R, Selwitz RH, Wu T. Surveillance for Dental Caries, Dental Sealants, Tooth Retention, Edentulism, and Enamel Fluorosis - United States, 1988-1994 and 1999-2002. Surveillance Summary, Dept of Health and Human Services, Center for Disease Control. 2005;54(03):1–44. [PubMed] [Google Scholar]
- Braem M, Lambrechts P, Vanherle G. Clinical relevance of laboratory fatigue studies. J Dent. 1994;22(2):97–102. doi: 10.1016/0300-5712(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Camargo MA, Marques MM, de Cara AA. Morphological analysis of human and bovine dentine by scanning electron microscope investigation. Arch Oral Biol. 2008;53(2):105–8. doi: 10.1016/j.archoralbio.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Carvajal-Carmona LG, Soto ID, Pineda N, Ortíz-Barrientos D, Duque C, Ospina-Duque J, McCarthy M, Montoya P, Alvarez VM, Bedoya G, Ruiz-Linares A. Strong Amerind/White Sex Bias and a Possible Sephardic Contribution among the Founders of a Population in Northwest Colombia. American Journal of Human Genetics. 2000;67(5):1287–1295. doi: 10.1016/s0002-9297(07)62956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Carmona LG, Ophoff R, Service S, Hartiala J, Molina J, Leon P, Ospina J, Bedoya G, Freimer N, Ruiz-Linares A. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Human Genetics. 2003;112(5-6):534–541. doi: 10.1007/s00439-002-0899-8. [DOI] [PubMed] [Google Scholar]
- Carvalho RM, Fernandes CA, Villanueva R, Wang L, Pashley DH. Tensile strength of human dentin as a function of tubule orientation and density. J Adhes Dent. 2001;3(4):309–314. [PubMed] [Google Scholar]
- Coutinho ET, Moraes d’Almeida JR, Paciornik S. Evaluation of microstructural parameters of human dentin by digital image analysis. Mater Res. 2007;10(2):153–159. [Google Scholar]
- Departamento Administrativo Nacional de Estadística (DANE) “Colombia una nación Multicultural. Su diversidad étnica”. Dirección de censos y demografía. Colombia, Mayo de 2007. (Colombia a multicultural nation. Its ethnic diversity. Cencus and Demography, Colombia, May, 2007)
- Dutra-Correa M, Anauate-Netto C, Arana-Chavez VE. Density and diameter of dentinal tubules in etched and non-etched bovine dentine examined by scanning electron microscopy. Arch Oral Biol. 2007;52(9):850–855. doi: 10.1016/j.archoralbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Estudio Nacional de Salud Bucal (ENSAB) Tomo VII, Ministerio de Salud, Republica de Colombia 1999. (National Study of Dental Health in Colombia, Volume 7, Ministry of Health, Republic of Colombia)
- Food and Agricultural Organization (FAO) of the United Nations. Perfiles Nutricionales por Paises– COLOMBIA. FAO Rome, Italy, 2001. (Nutritional profiles of countries – Colombia). http://www.fao.org/ag/agn/nutrition/col_es.stm.
- Ferracane JL. Resin composite--state of the art. Dent Mater. 2011;27(1):29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Fuentes V, Toledano M, Osorio R, Carvalho RM. Microhardness of superficial and deep sound human dentin. J Biomed Mater Res A. 2003;66(4):850–853. doi: 10.1002/jbm.a.10064. [DOI] [PubMed] [Google Scholar]
- Garberoglio R, Brannstrom M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21(6):355–362. doi: 10.1016/s0003-9969(76)80003-9. [DOI] [PubMed] [Google Scholar]
- Giannini M, Soares CJ, de Carvalho RM. Ultimate tensile strength of tooth structures. Dent Mater. 2004;20(4):322–329. doi: 10.1016/S0109-5641(03)00110-6. [DOI] [PubMed] [Google Scholar]
- Geurtsen W, Schwarze T, Günay H. Diagnosis, therapy, and prevention of the cracked tooth syndrome. Quintessence Int. 2003;34:409–417. [PubMed] [Google Scholar]
- Inoue S, Pereira PN, Kawamoto C, Nakajima M, Koshiro K, Tagami J, Carvalho RM, Pashley DH, Sano H. Effect of depth and tubule direction on ultimate tensile strength of human coronal dentin. Dent Mater J. 2003;22(1):39–47. doi: 10.4012/dmj.22.39. [DOI] [PubMed] [Google Scholar]
- Ivancik J, Neerchal NK, Romberg E, Arola D. On the reduction in fatigue crack growth resistance of dentin with depth. J Dent Res. 2011;90(8):1031–1036. doi: 10.1177/0022034511408429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancik J, Majd H, Bajaj D, Romberg E, Arola D. Contributions of aging to the fatigue crack growth resistance of human dentin. Acta Biomat. 2012;8(7):2737–2746. doi: 10.1016/j.actbio.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancik J, Arola D. The Importance of microstructural variations on the fracture toughness of human dentin. Biomaterials. 2013;34(4):864–874. doi: 10.1016/j.biomaterials.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JH, Balooch M, Marshall SJ, Marshall GW, Jr, Weihs TP. Atomic force microscope measurements of the hardness and elasticity of peritubular and intertubular human dentin. J Biomech Engr. 1996;118(1):133–135. doi: 10.1115/1.2795939. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14(1):13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- Koester KJ, Ager JW, 3rd, Ritchie RO. The effect of aging on crack-growth resistance and toughening mechanisms in human dentin. Biomaterials. 2008;29(10):1318–1328. doi: 10.1016/j.biomaterials.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Konishi N, Watanabe LG, Hilton JF, Marshall GW, Marshall SJ, Staninec M. Dentin shear strength: effect of distance from the pulp. Dent Mater. 2002;18(7):516–520. doi: 10.1016/s0109-5641(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Kruzic JJ, Nalla RK, Kinney JH, Ritchie RO. Mechanistic aspects of in vitro fatiguecrack growth in dentin. Biomaterials. 2005;26(10):1195–204. doi: 10.1016/j.biomaterials.2004.04.051. [DOI] [PubMed] [Google Scholar]
- Lopes MB, Sinhoreti MA, Gonini Júnior A, Consani S, McCabe JF. Comparative study of tubular diameter and quantity for human and bovine dentin at different depths. Braz Dent J. 2009;20(4):279–83. doi: 10.1590/s0103-64402009000400003. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25(6):441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- Mejía M. Secretaría de Salud certificó calidad del agua potable para consumo humano de 18 sistemas de acueductos urbano y rural. 2011. (Ministry of Health certified drinking water quality for 18 systems of urban and rural drinking water). http://www.medellin.gov.co/irj/portal/ciudadanos?NavigationTarget=navurl://97eb3d0fe095b5b8764849785de5c1fa.
- Mjör IA, Fejerskov O. Histology of the Human Tooth. 2nd ed. Copenhagen: Muksgaard; 1979. [Google Scholar]
- Mjör IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41(5):401–412. doi: 10.1016/0003-9969(96)00008-8. [DOI] [PubMed] [Google Scholar]
- Mjor IA, Toffeneti F. Secondary caries: a literature review with caries reports. Quintessence Int. 2000;31(3):165–179. [PubMed] [Google Scholar]
- Nalla RK, Imbeni V, Kinney JH, Staninec M, Marshall SJ, Ritchie RO. In vitro fatigue behavior of human dentin with implications for life prediction. J Biomed Mater Res A. 2003;66(1):10–20. doi: 10.1002/jbm.a.10553. [DOI] [PubMed] [Google Scholar]
- Nanci A. Ten Cate’s Oral Histology: Development, structure, and function. St. Louis: Mosby-Year Book, Inc.; 2008. [Google Scholar]
- Naranjo M. Resistencia a la fractura de dientes intactos y restaurados con resina sometidos a carga constante. Revista CES Odontología. 2007;20(2):31–38. [Google Scholar]
- Nazari A, Bajaj D, Zhang D, Romberg E, Arola D. On the reduction in fracture toughness of human dentin with age. J. Mech Behav Biomed Mater. 2009;2(5):550–559. doi: 10.1016/j.jmbbm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk G. Posteruptive effects of nutrition on teeth. J Dent Res. 1970;49(6):1252–62. doi: 10.1177/00220345700490061301. [DOI] [PubMed] [Google Scholar]
- Paris PC, Gomez MP, Anderson WP. A rational analytical theory of fatigue. Trend Eng. 1961;13:9–14. [Google Scholar]
- Pashley D, Okabe A, Parham P. The relationship between dentin microhardness and tubule density. Endod Dent Traumatol. 1985;1(5):176–9. doi: 10.1111/j.1600-9657.1985.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Pashley DH. Dentin: A dynamic substrate - a review. Scan Microsc. 1989;3:161–176. [PubMed] [Google Scholar]
- Ramirez RA. Incremento de volumen de cavidades clase I en molares humanos durante el reemplazo de restauraciones de resina compuesta y amalgama por diferentes grupos de operadores y su relación con el conocimiento en mio. Acta Odontológica Venezolana. 2008;43(3):289–294. [Google Scholar]
- Ramírez-Puerta BS, Franco-Cortés AM, Ochoa-Acosta EM. Dental fluorosis in 6-13- year-old children attending public schools in Medellín, Colombia. Rev Salud Publica (Bogota) 2009;11(4):631–40. doi: 10.1590/s0124-00642009000400014. [DOI] [PubMed] [Google Scholar]
- Roh BD, Lee YE. Analysis of 154 cases of teeth with cracks. Dental Traumatology. 2006;22:118–123. doi: 10.1111/j.1600-9657.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- Ryou H, Amin N, Ross A, Wang DH, Eidelman N, Romberg E, Arola D. Contributions of microstructure and chemical composition to the mechanical properties of dentin. J Mat Sci: Mater Med. 2011;22(5):1127–1135. doi: 10.1007/s10856-011-4293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21(1):9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Schilke R, Lisson JA, Bauss O, Geurtsen W. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch Oral Biol. 2000;45(5):355–61. doi: 10.1016/s0003-9969(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Shaw JH. Preeruptive effects of nutrition on teeth. J Dent Res. 1970;49(6):1238–51. doi: 10.1177/00220345700490061101. [DOI] [PubMed] [Google Scholar]
- Staninec M, Marshall GW, Hilton JF, Pashley DH, Gansky SA, Marshall SJ, Kinney JH. Ultimate tensile strength of dentin: Evidence for a damage mechanics approach to dentin failure. J Biomed Mater Res. 2002;63(3):342–5. doi: 10.1002/jbm.10230. [DOI] [PubMed] [Google Scholar]
- Tagami J, Tao L, Pashley DH, Horner JA. The permeability of dentine from bovine incisors in vitro. Arch Oral Biol. 1989;34(10):773–7. doi: 10.1016/0003-9969(89)90027-7. [DOI] [PubMed] [Google Scholar]
- Tesch W, Eidelman N, Roschger P, Goldenberg F, Klaushofer K, Fratzl P. Graded microstructure and mechanical properties of human crown dentin. Calcif Tissue Int. 2001;69(3):147–57. doi: 10.1007/s00223-001-2012-z. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, Population Estimates July 1, 2000 to July 1, 2006, Ethnicity and Ancestry Branch Population Division. 2006 www.census.gov/population/www/socdemo/hispanic.html.
- Watanabe LG, Marshall GW, Jr, Marshall SJ. Dentin shear strength: effects of tubule orientation and intratooth location. Dent Mater. 1996;12(2):109–15. doi: 10.1016/S0109-5641(96)80077-7. [DOI] [PubMed] [Google Scholar]
- Wegehaupt F, Gries D, Wiegand A, Attin T. Is bovine dentine an appropriate substitute for human dentine in erosion/abrasion tests? J Oral Rehabil. 2008;35(5):390–394. doi: 10.1111/j.1365-2842.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Noncommunicable diseases of Colombia. NCD Country profiles. Geneva, Switzerland: WHO Press; 2011. http://www.who.int/nmh/countries/col_en.pdf. [Google Scholar]
- Yahyazadehfar M, Ivancik J, Majd H, An B, Zhang D, Arola D. On the Mechanics of Fatigue and Fracture in Teeth. ASME Journal of Applied Mechanics Reviews. 2014 doi: 10.1115/1.4027431. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]