Abstract
Background
Physical exercise during leisure time is known to increase physical capacity; however, the long-term effects on work ability and work strain are inconclusive. The aim of this study was to investigate the effects of a 6-month physical exercise program on work ability and work strain after 6 months and 30 months, among women with menopausal symptoms at baseline.
Methods
A questionnaire including questions on work ability and work strain was mailed in the beginning, at 6 months and after 30 months after the intervention to occupationally active women participating in a randomized controlled study on physical exercise and quality of life. The intervention included aerobic exercise training 4 times per week, 50 minutes per session. Work ability was measured with the Work Ability Index (WAI) and with questions about physical and mental work strain.
Results
Women aged 47–62 years (N = 89) who were occupationally active at baseline were included in the analyses. The increase in WAI from baseline to the end of the exercise intervention (6 months) was statistically significantly greater among the intervention group than among the control group (regression coefficient 2.08; 95% confidence interval 0.71–3.46). The difference between the groups persisted for 30 months. No significant short- or long-term effects on physical and mental work strain were found.
Conclusion
A 6-month physical exercise intervention among symptomatic menopausal women had positive short-term as well as long-term effects on work ability.
Keywords: menopause, physical exercise intervention, randomized controlled trial, work ability, work strain
1. Introduction
Physical capacity and work ability decline with age because of biological and environmental factors [1,2]. Decline in aerobic (physical) capacity is on average 5–15% per decade between the ages of 30 years and 60 years [3]. Lifestyle factors (e.g., age, sex, and physical activity level) may affect the decline of physical capacity, but the effects of age can mainly been seen after the age of 45 years [4,5]. Physical capacity could be improved through increased physical activity at all ages. All workers, especially middle-aged workers, benefit from physical training, and beneficial effects on work-related outcomes have also been reported [6,7]. Chronic disease, poor musculoskeletal fitness, and cardiorespiratory fitness are associated with low physical capacities, whereas well-being, physical demands of the work [8], and poor work ability [9] [measured with the Work Ability Index (WAI)] decline with age. WAI in women is slightly lower than in men. Especially during the transitional ages of menopause, the differences between men and women are greater but are smaller in older age groups, which may be a sign of the effect of the menopause [10,11]. Decreased work ability in most women may be caused by sex-specific age-related diseases, and lack of mental resources, partly because of commitments outside work and more sickness absence [1].
It is known that good physical fitness is associated with well-being and health at many levels [12]. Better physical capacity may increase work ability and help people to cope with physical strain at work. It could also improve mental resources; help to recover faster from mental strain, and positively affect work ability. Information about the effects of physical exercise on work-related factors is, however, limited and inconsistent [12].There are only a few well-designed studies of the effectiveness of leisure-time physical exercise programs on work-related outcomes [12,13]. Most of the interventions are worksite related and have focused on the effect of exercise in either physical or mental occupations [9]. The effect has been very limited, e.g., regarding sickness absence, inconclusive for job satisfaction, job stress, and employee turnover, and nil for productivity. The viewpoint of work ability and strain at work has received little attention. A physical exercise intervention that included mental health workers, police officers, homecare workers, and cleaners, showed only a slight improvement in work ability despite a significant change in physical capacity [7,14–17].
It is not known whether or not menopause symptoms have an effect on work ability. The menopausal transition may affect quality of life and also the ability to cope with increasing work demands, because up to 80% of women report menopause-related symptoms [18,19], such as hot flushes, sleep disturbances, and mood swings [20,21]. The most effective treatment for menopausal symptoms is hormone therapy, but the risks may outweigh the benefits [17]; thus, alternative treatments are warranted. An increase in physical exercise is known to have beneficial effects on overall health [22,23] and could therefore also alleviate menopause-related symptoms, and also result in improved work ability. There is a lack of information on the effect of the menopausal transition on work ability and work-related factors [24]. We found only one cross-sectional study suggesting that menopausal symptoms are negatively associated with work ability and may increase the risk of sickness absence [11]. However, it is known that menopausal symptoms (transition) have an effect on those health- and well-being–related factors, which are associated with work ability and work-related factors [25].
We have reported earlier on the primary outcomes of the randomized controlled 6-month exercise trial used in this study. These were improved quality of life and decreased menopausal symptoms [26,27]. We have also reported the secondary results related to perceived work strain, and work ability among all employed, responding women [28]. The aim of this study was to include women in employment and to study both the short- and long-term effects (30 months) of the 6-month physical exercise on perceived work ability and work strain.
2. Materials and methods
2.1. Study design
The study was a secondary analysis of a randomized controlled study [26] with 2-year follow-up. The main outcome measures were perceived work ability and work strain. In this study, only those who were occupationally active (working > 1 h/wk), continued their participation until the end of the intervention, and completed all questionnaires were included in the analyses (n = 89). The study participants were mostly working in mentally demanding jobs (n = 65; e.g., office work), but also in positions requiring physical activity (n = 5; e.g., cleaning) and mixed (physical and mental) jobs (n = 19; e.g., nursing).
Women were recruited via local newspapers. Details of the recruitment and participant selection were reported earlier [26]. One hundred and seventy-six menopausal women participated in the study. Inclusion criteria were: symptomatic (daily hot flushes), age 40–62 years, no current postmenopausal hormone therapy, or hormonal therapy withdrawal (washout period 3 months), low physical activity (physical exercise < 3 times/week) and 6–36 months since their last menstruation.
The duration of the intervention was 6 months. The women were randomized into intervention and control groups. The intervention included aerobic exercise training four times per week, 50 minutes per session, with a progressive increase in intensity. At least two sessions were to involve walking or Nordic walking and the other two could be jogging, cycling, swimming, skiing, aerobics, or other gymnastic exercise. Walking was emphasized because of the experiences from earlier trials as having favorable effects on health among menopausal and postmenopausal women [29]. Adherence to the trial was supported by an option to participate in supervised aerobics or step aerobics sessions at the research institute twice per week. The ratings of perceived exertion were used to check the intensity of the exercise. The target for the rated value during the exercise was 13–16 on a 6–20 point scale. This corresponds to about 64–80% of the maximum heart rate [30]. The control group was asked to maintain their normal physical activity habits. Both the intervention and control groups attended lectures once or twice per month (6–12 times/6 months). The lectures took 60–75 minutes and mostly addressed topics of physical activity and general health [26].
Twenty-four months after the physical exercise intervention we mailed a follow-up questionnaire to all study participants (n = 176), of whom 102 (57%) answered the questionnaire. All the study participants provided written consent and the study was approved by the Pirkanmaa Hospital District Ethics committee (Tampere, Finland).
2.2. Assessments
Questionnaires were completed on paper at baseline, after 6 months and 30 months from baseline. The WAI was used to estimate perceived work ability [2]. The final WAI score comprises the sum of seven items: (1) work ability in relation to lifetime best (scale 0–10); (2) work ability in relation to physical and mental work demands (scale 1–5); (3) number of diagnosed diseases; (4) estimation of work impairment caused by diseases (scale 1–6); (5) self-reported sick leaves during the past 12 months (scale 1–5); (6) personal prognosis of work ability after 2 years (scale 1, 4, and 7); and (7) mental resources (scale 1–4). The WAI score could range from 7 to 49 (poor to excellent).
Physical and mental strain at work was elicited using a modified Borg scale (How much physical/mental strain do you feel on a normal work day? (from 0 = very little to 10 = very much) [30]. Cardiorespiratory fitness was assessed by the 2-km Urho Kaleva Kekkonen (UKK) Walking Test. The UKK Walking Test is a reliable method for measuring aerobic fitness for 20–65-year-old adults with no illness or disability limiting brisk walking or who are not taking medication that affects heart rate [31]. Heart rate was monitored during the walk and registered immediately at the end (Polar Electro, Oulu, Finland). Maximal oxygen consumption was estimated through a formula based on a sex-specific model including walking time, heart rate at the end of the walk, age, and body mass index [31].
2.3. Statistical analysis
Characteristics of the study participants are described using means and standard deviations or proportions. Cross-sectional differences between groups at baseline and at 6 month and 30 month follow-up were evaluated using the Mann-Whitney U test. Normality of the continuous variables was evaluated with the Kolmogorov-Smirnov test.
To account for the within-participant correlation between three time points, we constructed multilevel ordinal logistic regression models (called the proportional odds model) and analyzed the association of outcomes (work ability index, physical and mental strain) over time by group (intervention/control). Two-level models consisted of fixed effects (group, follow-up time in months, interaction between these variables, age of the respondent, and work demands) and random effects (measurements within participants). Models included a variable (group) to indicate the difference between groups at baseline and a variable (time) to indicate the change of outcome over time. The difference in the change in outcome variables across the three time points between groups was tested using an interaction term between group and follow-up time. The parameter estimates were presented with 95% confidence intervals and p values. The proportional odds assumption of the ordinal regression was tested by comparing proportional and nonproportional odds models using the likelihood ratio test [32].
Data showed that participants who were lost to follow-up were similar in all background variables when compared with participants included in the analysis.
Cross-sectional analyses were performed using SPSS software (IBM SPSS Statistics version 20, IBM, USA) and multilevel models were constructed by generalized linear latent and mixed models command using STATA software (version 12.0 for Windows, StataCorp, USA).
3. Results
At baseline there were no other statistical differences between the groups except in the work demands: women in the intervention group did mental work less often (65.1%) than women in the control group (81.0%; Table 1). The working hours of the women varied between 4 hours and 70 hours per week (mean 34.3, standard deviation 15.7), and a fourth of the women had a university degree.
Table 1.
Baseline characteristics of the intervention and control groups (mean and standard deviations or proportions)
| Intervention group (n = 45) | Control group (n = 44) | |
|---|---|---|
| Age (y) | 54.8 ± 3.3 | 54.1 ± 3.2 |
| Height, (cm) | 163.9 ± 5.7 | 163.2 ± 5.5 |
| Weight (kg) | 68.9 ± 9.1 | 71.8 ± 12.8 |
| BMI (kg/m2) | 25.7 ± 3.8 | 26.9 ± 4.1 |
| VO2max (ml/kg/min) | 31.5 ± 6.9 | 31.4 ± 4.1 |
| Work (h/wk) | 32.7 ± 14.9 | 36.0 ± 16.4 |
| Work demands | ||
| Physical | 9.3 | 2.4 |
| Mixed | 25.6 | 16.7 |
| Mental | 65.1 | 81.0 |
| Education | ||
| Low | 31.1 | 29.5 |
| Middle | 42.2 | 43.2 |
| High | 26.7 | 27.3 |
| Work ability index | 38.3 ± 7.2 | 38.7 ± 5.4 |
| Physical strain at work | 3.8 ± 3.0 | 4.6 ± 2.7 |
| Mental strain at work | 6.5 ± 2.3 | 6.1 ± 2.3 |
Data are presented as % or mean ± SD.
Work ability index was originally lower in the intervention group than in the control group, but during the intervention the WAI increased in the intervention group but decreased in the control group (Fig. 1). At the end of the 6-month intervention the difference between the randomized groups was statistically significant (p < 0.05) and the difference between the groups persisted after adjusting for age and work demands (Table 2). There were no significant differences in changes of physical and mental strain between the randomized groups.
Fig. 1.
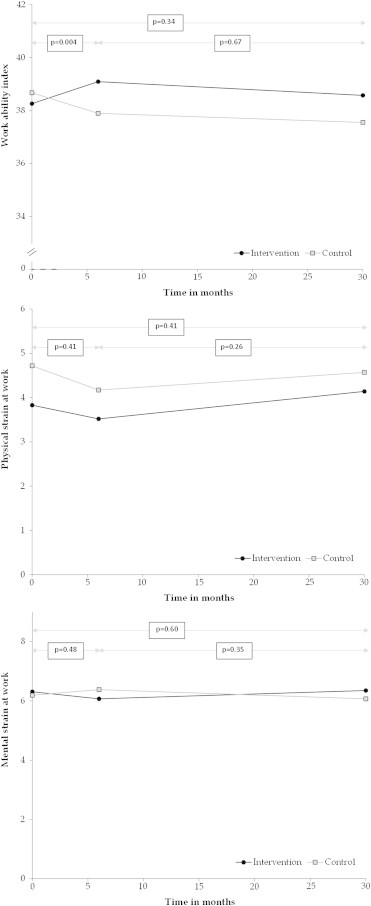
Work Ability Index physical and mental work strain in timeline between the intervention and control group (n = 70–85).
Table 2.
Linear regression for changes 0–6 months and 6–30 months and the proportional odds models adjusted for age and work demands using the work ability index and other work related factors as responses (n = 84–85)
| Change 0–6 mo |
Change 6–30 mo |
Change 0–30 mo (all 3 timepoints)∗ |
|||
|---|---|---|---|---|---|
| Group |
Group |
Group (baseline) |
Time |
Group∗ Time |
|
| Beta (95% CI) | Beta (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Work ability index | 2.08 (0.71–3.46) | −0.49 (−2.76–1.79) | 1.65 (0.20–13.5) | 0.98 (0.93–1.03) | 1.03 (0.97–1.11) |
| Physical strain at work | −0.40 (−1.34–0.55) | −0.57 (−1.57–0.43) | 0.26 (0.07–1.04) | 1.01 (0.98–1.04) | 1.02 (0.98–1.06) |
| Mental strain at work | −0.29 (−1.08–0.51) | 0.39 (−0.43–1.20) | 1.55 (0.35–6.89) | 0.99 (0.96–1.02) | 1.01 (0.97–1.06) |
CI, confidence interval; OR, odds ratio.
Linear models are adjusted for baseline, age, and work demands; multilevel models were adjusted for age and work demands.
However, the estimated difference in the slopes between the groups was not significant when all three time points were considered (Table 2), which may be caused by the opposite directions of change during the intervention period.
The estimated odds of higher levels of WAI for a study participant in the control group were multiplied by 0.98 every month and the odds for a study participant in the intervention group were multiplied by 1.01 (0.98 × 1.03) every month. The interaction (group*time) was more likely to show an improvement in work ability among participants in the intervention group than in the control group. However, the estimated differences in the slopes of time between the two groups were not significant at the 5% level. In other characteristics there were no differences between the groups when all three time points were included in the analyses.
4. Discussion
The results showed that the 6-month physical exercise intervention program had both short- and long-term effects on the menopausal women's work ability, but no significant effects on physical and mental strain during work. After 30 months, work ability and work strain had decreased in both groups, but the work ability was still higher than at baseline.
The work ability index declines 0.8 points per year among women between the age of 45 years and 58 years, without any known intervention [2]. In our study WAI was almost on the same level after 30 months, which could be deemed as a positive outcome. Other studies have reported limited longitudinal effects of physical exercise interventions on work ability and work related-factors [14]. In a 36-month worksite physical activity intervention among women in the social sector, women older than 45 years reported improvement only in their future work expectations, whereas younger women reported significant improvement in their work ability and general health [16].
As shown previously, good physical fitness is related to better work ability among aging workers [6,9,33]. Our study showed positive short-time effects and sustaining long-term effects on work ability but no effects on perceived work strain. This may in part be because of the small sample size but more probably because the intervention was not focused on work itself during the intervention period. In addition, in our study only about 10% of the participants in both groups were workers in physically demanding occupations. The physical exercise intervention might have been more effective, at least according to perceived physical work strain, if all the participants had worked in more physically demanding occupations, where good physical capacity is essential to meet the physical demands at work.
Menopausal symptoms are an important confounding factor for work ability. Psychological well-being in menopausal women is closely associated with current health and lifestyle variables [20]. However, one study concerning the timing of menopause and job control suggests that premature menopause is associated with high job control and high formal education [34], suggesting that certain physical job stressors may be related to age at menopause. The effects of menopause on women's work ability has not been widely studied.
The most important strength of our study was the prospective study setting with a group of symptomatic menopausal women. Our participants also showed good compliance: 70% of the working study participants participated in 30-month follow-up. However, our study also has some limitations. Work ability and work-related factors were not the main outcomes in the original randomized trial, as was quality of life. The women worked mainly in mentally demanding occupations. Some older participants were over the menopause transition at the 30-month follow-up.
In conclusion a 6-month physical exercise intervention among working symptomatic menopausal women had positive short-term as well as long-term effects on work ability.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Juho Vainio Foundation, the Ministry of Education and Culture, and the Pirkanmaa Competitive Research Fund (Pirkanmaa Hospital District).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Gould R., Ilmarinen J., Järvisalo J., Koskinen S. Dimension of work ability—summary and conclusion. In: Gould R., Ilmarinen J., Järvisalo S., Koskinen S., editors. Dimension of work ability: results of the Health 2000 Survey. Finnish Centre of Pensions, The Social Insurance Institution, National Public Health Institute, Finnish Institute of Occupational Health; Helsinki: 2008. [Google Scholar]
- 2.Ilmarinen J., Tuomi K., Klockars M. Changes in the work ability of active employees as measured by the work ability index over an 11-year period. Scand J Work Environ Health. 1997;23:49–57. [PubMed] [Google Scholar]
- 3.Shwartz E., Reibold R.C. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61:3–11. [PubMed] [Google Scholar]
- 4.Fleg J.L., Morrell C.H., Bos A.G., Brant L.J., Talbot L.A., Wright J.G., Lakatta E.G. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 5.Jackson A.S., Sui X., Hébert J.R., Church T.S., Blair S.N. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–1787. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenny G.P., Yardley J.E., Martineau L., Jay O. Physical work capacity in older adults: implications for the aging worker. Am J Ind Med. 2008;51:610–625. doi: 10.1002/ajim.20600. [DOI] [PubMed] [Google Scholar]
- 7.Pohjonen T. Age-related physical fitness and the predictive values of fitness tests for work ability in home care work. J Occup Environ Med. 2001;43:723–730. doi: 10.1097/00043764-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Gram B., Holtermann A., Bültmann U., Sjøgaard G., Søgaard K. Does an exercise intervention improving aerobic capacity among construction workers also improve musculoskeletal pain, work ability, productivity, perceived physical exertion, and sick leave?: a randomized controlled trial. J Occup Environ Med. 2012;54:1520–1526. doi: 10.1097/JOM.0b013e318266484a. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg T.I., Elders L.A., de Zwart B.C., Burdorf A. The effects of work-related and individual factors on the Work Ability Index: a systematic review. Occup Environ Med. 2009;66:211–220. doi: 10.1136/oem.2008.039883. [DOI] [PubMed] [Google Scholar]
- 10.Tuomi K., Ilmarinen J., Martikainen R., Aalto L., Klockars M. Aging, work, life-style and work ability among Finnish municipal workers in 1981–1992. Scand J Work Environ Health. 1997;23:58–65. [PubMed] [Google Scholar]
- 11.Geukes M., van Aalst M.P., Nauta M.C., Oosterhof H. The impact of menopausal symptoms on work ability. Menopause. 2012;19:278–282. doi: 10.1097/gme.0b013e31822ddc97. [DOI] [PubMed] [Google Scholar]
- 12.Penedo F.J., Dahn J.R. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Ilmarinen J. Gummerus Kirjapaino Oy; Jyväskylä: 2006. Towards a longer worklife: ageing and the quality of worklife in the European Union. [Google Scholar]
- 14.Smolander J., Blair S.N., Kohl H.W. Work ability, physical activity, and cardiorespiratory fitness: 2-year results from Project Active. J Occup Environ Med. 2000;42:906–910. doi: 10.1097/00043764-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Sjögren T., Nissinen K.J., Järvenpää S.K., Ojanen M.T., Vanharanta H., Mälkiä E. Effects of a physical exercise intervention on subjective physical well-being, psychosocial functioning and general well-being among office workers: a cluster randomized-controlled cross-over design. Scand J Med Sci Sports. 2006;16:381–390. doi: 10.1111/j.1600-0838.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 16.Vingård E., Blomkvist V., Rosenblad A., Lindberg P., Voss M., Alfredsson L., Josephson M. A physical fitness programme during paid working hours - impact on health and work ability among women working in the social service sector: a three year follow up study. Work. 2009;34:339–344. doi: 10.3233/WOR-2009-0932. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., Jackson R.D., Beresford S.A., Howard B.V., Johnson K.C., Kotchen J.M., Ockene J., Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Freeman E.W., Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10:197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 19.Moilanen J., Aalto A.M., Hemminki E., Aro A.R., Raitanen J., Luoto R. Prevalence of menopause symptoms and their association with lifestyle among Finnish middle-aged women. Maturitas. 2010;67:368–374. doi: 10.1016/j.maturitas.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Samsioe G., Dören M., Lobo R.A. Acute symptoms of the menopause. Women's Health Med. 2006;3:282–286. [Google Scholar]
- 21.Dennerstein L. Well-being, symptoms and the menopausal transition. Maturitas. 1996;23:147–157. doi: 10.1016/0378-5122(95)00970-1. [DOI] [PubMed] [Google Scholar]
- 22.Aiello E.J., Yasui Y., Tworoger S.S., Ulrich C.M., Irwin M.L., Bowen D., Schwartz R.S., Kumai C., Potter J.D., McTiernan A. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause. 2004;11:382–388. doi: 10.1097/01.gme.0000113932.56832.27. [DOI] [PubMed] [Google Scholar]
- 23.Wilbur J.E., Miller A.M., McDevitt J., Wang E., Miller J. Menopausal status, moderate-intensity walking and symptoms in midlife women. Res Theory Nurs Pract. 2005;19:163–180. [PubMed] [Google Scholar]
- 24.Hammam R.A., Abbas R.A., Hunter M.S. Menopause and work–the experience of middle-aged female teaching staff in an Egyptian governmental faculty of medicine. Maturitas. 2012;71:294–300. doi: 10.1016/j.maturitas.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Woods N.F., Mitchell E.S. Symptom interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2011;18:654–661. doi: 10.1097/gme.0b013e318205bd76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luoto R., Moilanen J., Heinonen R., Mikkola T., Raitanen J., Tomas E., Ojala K., Mansikkamäki K., Nygård C.H. Effect of aerobic training on hot flushes and quality of life – a randomized controlled trial. Ann Med. 2012;44:616–626. doi: 10.3109/07853890.2011.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moilanen J.M., Mikkola T.S., Raitanen J.A., Heinonen R.H., Tomas E.I., Nygård C.H., Luoto R.M. Effect of aerobic training on menopausal symptoms–a randomized controlled trial. Menopause. 2012;19:691–696. doi: 10.1097/gme.0b013e31823cc5f7. [DOI] [PubMed] [Google Scholar]
- 28.Rutanen R., Nygård C.H., Moilanen J., Mikkola T., Raitanen J., Tomas E., Luoto R. Effect of physical exercise on work ability and daily strain in symptomatic menopausal women: a randomized controlled trial. Work. 2014;47:281–286. doi: 10.3233/WOR-121586. [DOI] [PubMed] [Google Scholar]
- 29.Asikainen T.M., Miilunpalo S., Oja P., Rinne M., Pasanen M., Uusi-Rasi K., Vuori I. Randomized, controlled walking trials in postmenopausal women: the minimum dose to improve aerobic fitness? Br J Sports Med. 2002;36:189–194. doi: 10.1136/bjsm.36.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehab Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 31.Oja P., Laukkanen R., Pasanen M., Tyry T., Vuori I. A 2-km walking test for assessing the cardiorespiratory fitness of healthy adults. Int J Sports Med. 1991;12:356–362. doi: 10.1055/s-2007-1024694. [DOI] [PubMed] [Google Scholar]
- 32.Rabe-Hesketh S., Skrondal A. 2nd ed. Stata Press; College Station, Texas: 2008. Multilevel and longitudinal modeling using STATA. [Google Scholar]
- 33.Mackey M., Maher C.G., Wong T., Collins K. Study protocol: the effects of work-site exercise on the physical fitness and work-ability of older workers. BMC Musculoskel Disord. 2007;8:9. doi: 10.1186/1471-2474-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassou B., Mandereau L., Aegerter P., Touranchet A., Derriennic F. Work-related factors associated with age at natural menopause in a generation of French gainfully employed women. Am J Epidemiol. 2007;166:429–438. doi: 10.1093/aje/kwm104. [DOI] [PubMed] [Google Scholar]


