Graphical abstract

Keywords: Schistosoma, Schistosomiasis, Praziquantel, ABC transporters, Drug resistance, Excretion, Reproduction, Parasite–host interactions
Highlights
-
•
The genuine and hypothesized roles of schistosome ABC transporters are reviewed.
-
•
Evidence suggesting a role for transporters in schistosome drug susceptibility is discussed.
-
•
Potential roles of ABC transporters in normal schistosome biology are outlined.
Abstract
Praziquantel (PZQ) is essentially the only drug currently available for treatment and control of schistosomiasis, a disease affecting hundreds of millions worldwide. Though highly effective overall, PZQ has limitations, most notably its significant lack of activity against immature schistosomes. Furthermore, the availability of only a single drug for a disease of this magnitude makes reports of PZQ-resistant isolates particularly troubling. ATP-binding cassette (ABC) multidrug transporters such as P-glycoprotein (Pgp; ABCB1) are efflux transporters that underlie multidrug resistance (MDR); changes in their expression or structure are also associated with drug resistance in parasites, including helminths. This review will discuss the role these transporters might play in modulating schistosome susceptibility to PZQ, and the implications for developing new or repurposed treatments that enhance the efficacy of PZQ. However, in addition to influencing drug susceptibility, ABC transporters play important roles in several critical physiological functions such as excretion and maintenance of permeability barriers. They also transport signaling molecules with high affinity, and several lines of evidence implicate mammalian transporters in a diverse array of physiological functions, including regulation of immune responses. Like their mammalian counterparts, schistosome ABC transporters appear to be involved in functions critical to the parasite, including excretory activity and reproduction, and we hypothesize that they underlie at least some aspects of parasite–host interactions. Thus, in addition to their potential as targets for enhancers of PZQ susceptibility, these transporters might also serve as candidate targets for agents that disrupt the parasite life cycle and act as antischistosomals on their own.
1. Introduction
Efflux transporters of the ATP binding cassette (ABC) protein superfamily mediate multidrug resistance (MDR), and are also associated with drug resistance in parasites, including helminths (reviewed in Leprohon et al., 2011; Kasinathan and Greenberg, 2012; Lespine et al., 2012; Ardelli, 2013; Greenberg, 2013a). As ABC transporters can serve as important regulators of drug susceptibility, they are excellent candidate targets for inhibitors that act as adjuncts to current anthelmintics to enhance effective potency. Indeed, several laboratories have been exploring the possibility of increasing anthelmintic effectiveness and overcoming drug resistance by repurposing currently-available drugs that can inhibit ABC transporters. This exciting approach, which could provide new strategies for combination therapy, has recently been reviewed extensively (James et al., 2009a,b; Ardelli, 2013; Greenberg, 2013a,b; Lespine et al., 2012). This review will also briefly discuss this strategy, but will focus primarily on the possible physiological functions of ABC transporters within schistosomes, and the potential for their exploitation as therapeutic targets on their own.
2. Schistosomes, schistosomiasis, and the need for new therapeutics
Schistosomiasis, caused by parasitic flatworms of the genus Schistosoma, is a major neglected tropical disease that constitutes a significant health and economic burden for hundreds of millions of people (van der Werf et al., 2003; King, 2010). Schistosome infections can produce severe damage to various organs, significant morbidity, impair childhood development and adult productivity, potentially increase susceptibility to other infections such as HIV, and, in some cases, lead to death (van der Werf et al., 2003; King and Dangerfield-Cha, 2008; Hotez and Fenwick, 2009; King, 2010; Ndeffo Mbah et al., 2013; Colley et al., 2014). There is at present no vaccine. Infrastructural and educational interventions to reduce transmission and prevent infection can be highly effective (Tanaka and Tsuji, 1997), but are expensive and require levels of organization that are often impractical in the developing world. Use of molluscicides to eliminate the intermediate host snails raises environmental concerns, can be expensive, and often produces only limited, short-term effectiveness (Sturrock, 2001).
For the past several years, both treatment and control of schistosomiasis have relied on chemotherapy with praziquantel (PZQ), the current drug of choice and essentially the only antischistosomal drug commercially available. Dependence on a single drug would be inadvisable for any infectious disease, but is particularly troubling for one at such high prevalence (Caffrey, 2007). PZQ has been available for several decades, and the history of its development and use has been reviewed extensively (Andrews et al., 1983; Redman et al., 1996; Cioli and Pica-Mattoccia, 2003; Doenhoff and Pica-Mattoccia, 2006; Doenhoff et al., 2008; Chai, 2013; Cioli et al., 2014). The main advantages of PZQ include its effectiveness against all human schistosome species, its comparatively mild side effects, and, in more recent years, its relatively low cost (Ndeffo Mbah et al., 2013). Large-scale PZQ treatment programs have produced significant reductions in disease prevalence and intensity (Vennervald et al., 2005; Toure et al., 2008; Sesay et al., 2014); indeed, based on this success, Merck has committed to donating 250 million tablets of PZQ annually for mass drug administration by 2016.
Nonetheless, PZQ does have significant shortcomings. Most notably, schistosomes show major stage-specific differences in PZQ susceptibility; immature worms (2–4 weeks post infection) exhibit insusceptibility to PZQ, making treatment largely ineffective until egg production begins approximately 5–6 weeks post-infection (Xiao et al., 1985; Sabah et al., 1986; Pica-Mattoccia and Cioli, 2004; Aragon et al., 2009). Though typical failure rates for PZQ are in the 5–30% range, certain regions and age groups show lower levels of efficacy, with failure rates often reaching 30–50% (Day and Botros, 2006; King et al., 2011; Mutapi et al., 2011; Sousa-Figueiredo et al., 2012; Garba et al., 2013; Tukahebwa et al., 2013). Furthermore, several field isolates exhibit further reduced susceptibility to PZQ, and PZQ resistance can be experimentally induced in schistosomes (reviewed by Day and Botros, 2006; Doenhoff and Pica-Mattoccia, 2006; Wang et al., 2012; Greenberg, 2013b). Though there is at present no credible evidence for emergence of widespread PZQ resistance, it remains a distinct possibility, particularly as mass drug administration programs are intensified. Furthermore, the mode of action of PZQ has yet to be defined rigorously. PZQ disrupts Ca2+ homeostasis in the parasite, with resultant paralysis and tegumental disruption, and several lines of evidence point to voltage-gated Ca2+ channels as important mediators of PZQ activity (Greenberg, 2005a; Chan et al., 2012, 2014). Nonetheless, the precise identity of the primary “PZQ receptor” remains unresolved (Redman et al., 1996; Doenhoff et al., 2008; Cioli et al., 2014), confounding rational approaches that might target similar pathways for increased potency or to overcome drug resistance.
Defining the molecular target of PZQ is obviously essential, but will likely not fully illuminate how PZQ works. Thus, several lines of evidence point to a role for downstream factors that influence parasite susceptibility to the drug. The most obvious example can be found in the difference in PZQ responsiveness between adult and juvenile worms. As in adults, PZQ induces a rapid, Ca2+-dependent contraction and paralysis of juvenile worms (Pica-Mattoccia et al., 2008). However, unlike adult worms, which remain paralyzed and ultimately die, the juveniles recover. This remarkable phenomenon suggests that though adults and juveniles share the PZQ molecular target and initial outputs (e.g., Ca2+ influx, perhaps via Ca2+ channels, contraction and paralysis); juveniles, but not adults, can marshal downstream protective responses that allow them to survive and recover. Indeed, gene expression profiling of adult and juvenile worms following exposure to PZQ supports the notion that these developmental stages differ in the way they respond to the drug (Hines-Kay et al., 2012).
As we and others have discussed, combination therapy, in which current anthelmintics are potentiated by new or repurposed agents that target different, but possibly interacting, sites of action, represents a potentially powerful strategy for overcoming drug resistance and enhancing drug efficacy (Geary et al., 2010; Hu et al., 2010; Greenberg, 2013b). P-glycoprotein (Pgp) and other ATP binding cassette (ABC) efflux transporters have been proposed as particularly attractive targets for this type of approach (Liang and Aszalos, 2006; Lespine et al., 2008). Several recent reviews have summarized the evidence that ABC multidrug transporters modulate helminth drug susceptibility (and resistance) and have explored their potential as targets to enhance anthelmintic activity (Leprohon et al., 2011; Kasinathan and Greenberg, 2012; Lespine et al., 2012; Ardelli, 2013; Greenberg, 2013a). What is particularly appealing about such a strategy is that many inexpensive drugs already in clinical use are known to interact with (i.e., inhibit) these transporters, providing the potential for repurposing these compounds for use in an anthelmintic treatment and control strategy. This type of approach is under intensive investigation in several labs.
On the other hand, ABC transporters are part of an ancient superfamily of proteins, and clearly have physiological functions beyond regulating susceptibility to modern drugs. This review will focus on those possible functions in schistosomes, with an eye towards how they may be exploited to disrupt the parasite life cycle or reduce disease pathology and transmission.
3. ABC transporters
ABC transporters are members of the ABC protein superfamily, a large, ancient group of proteins with representation in all kingdoms of life (Dassa and Bouige, 2001; Borst and Elferink, 2002). The common defining feature of ABC transporters is that they contain nucleotide binding domains (NBDs) that bind ATP (the ATP binding cassettes), and use the energy resulting from ATP hydrolysis to translocate compounds across the membrane. Some prokaryotic ABC transporters import compounds into the cell; efflux ABC transporters are found in both prokaryotes and eukaryotes (Dassa and Bouige, 2001; Saier and Paulsen, 2001). The different ABC transporters have selectivity for various substrates, ranging from metabolic byproducts to physiologically significant signaling molecules such as peptides, lipids, cyclic nucleotides, and immunomodulators (see Table 1). A subset of these transporters (ABCB1, or Pgp, ABCG2, several members of the ABCC sub-family, and possibly ABCA2) have been linked to multidrug resistance (MDR; Dean et al., 2001; Szakacs et al., 2006). MDR results from an increased capacity for efflux of both the original drug as well as other unrelated compounds via amplification, overexpression, or modification of these transporters.
Table 1.
Examples of potential signaling molecules that are substrates of ABC transporters.
| Compound | Functions | Transporter(s) | References |
|---|---|---|---|
| Platelet activating factor (PAF) | Mediator of inflammation | ABCB1 (Pgp) | Ernest and Bello-Reuss (1999), Raggers et al. (2001) |
| Phospholipids [e.g., phosphatidylcholine, phosphatidylserine (PS) phosphatidylethanolamine] | Membrane integrity, cell cycle regulation; cell signaling; schistosome PS and lyso-PS polarize DCs | ABCB1, ABCB3, ABCA1, ABCA2, ABCG2 (BCRP) | Pohl et al. (2002), Aye et al. (2009), Romsicki and Sharom (2001), Daleke (2007) |
| Cyclic nucleotides | Regulate inflammatory responses, monocyte polarization, maturation | ABCC4, ABCC5, ABCC11 | Wielinga et al. (2003), Chen et al. (2001), Guo et al. (2003) |
| Sphyngosine-1-phosphate (S1P) | T-cell homing; immunosuppression | ABCB1 | Honig et al. (2003) |
| Leukotriene LTC4 | Mediator of inflammation; DC migration | ABCC1, other ABCCs (MRPs) | Leier et al. (1994), Cui et al (1999), Rius et al. (2008) |
| LTB4, LTD4, LTE4 | Mediators of inflammation; DC migration | ABCC1, ABCC4 | Leier et al. (1994), Rius et al. (2008) |
| Prostaglandins PGE1, PGE2, PGE2α | DC migration/maturation; immune suppression | ABCC4 | Reid et al. (2003) |
| Sphingomyelin, glycolipids, cholesterol | Multiple | ABCB1, ABCAs | Kim et al. (2008) |
| Peptides | Antigen presentation | ABCB2/3 (TAP1/2) | Koopmann et al. (1996) |
| dsRNA | TLR3 activation in DCs by schistosome eggs | C. elegans ABCA, ABCBs, ABCD, ABCGs | Sundaram et al. (2006) |
Examples of some of the signaling molecules shown to be substrates of ABC transporters, and their possible functions, with an emphasis on those with relevance to immunomodulation.
By definition, ABC transporters contain at least one cytoplasmic ATP binding cassette, a highly-conserved ATPase domain containing a specific signature motif linked to WalkerA and WalkerB motifs that are characteristic of ATPases (Hyde et al., 1990). Some of the best-studied ABC transporters contain two ATP-binding cassettes alternating with two membrane-spanning domains (e.g., Pgp); these are considered full transporters, while those containing a single domain are classified as half transporters (Ambudkar et al., 2003; Sheps et al., 2004; Szakacs et al., 2006). The various ABC transporters cluster into eight families, designated ABCA through ABCH. Members of two of these families (ABCE and ABCF) contain two NBDs, but no transmembrane domains. Although they are not known to exhibit any transporter function, their NBDs appear to be derived from other ABC transporters, and they are therefore included with the transporters (Dean et al., 2001). These variations on ABC transporter domain structure are depicted in Fig. 1A. In humans, 48 ABC transporter genes representing seven of these families (ABCA to ABCG) have been identified, while representatives of all eight families can be found in the Drosophila and zebrafish genomes (Dean and Allikmets, 2001; Dean and Annilo, 2005; Annilo et al., 2006). Schistosome genomes code for approximately 20 transporters in 6 of these 8 sub-families; to date, no genes for ABCD or ABCH transporters have been detected (Greenberg, 2013a). As in parasitic and free-living nematodes (Ardelli, 2013), the majority of ABC transporters in Schistosoma mansoni (seven) are from the ABCB class, and most of those appear to be Pgp (ABCB1)-like. S. mansoni appears to have four genes encoding Pgp-like proteins, at least four genes of the ABCC class (multidrug resistance associated proteins; MRPs), including two MRP1 orthologues, and two orthologues of ABCG2 (breast cancer resistance protein; BCRP). Other potentially interesting ABC transporter genes include three that appear to be members of the ABCA family, lipid transporters implicated in neurodegenerative disorders (Piehler et al., 2012). Similar to the situation in nematodes, there is evidence of a reduction in overall number of genes in the parasitic worms; the free-living planarian Schmidtea meditteranea (Robb et al., 2008) appears to have 10–15 Pgp genes in its genome.
Fig. 1.
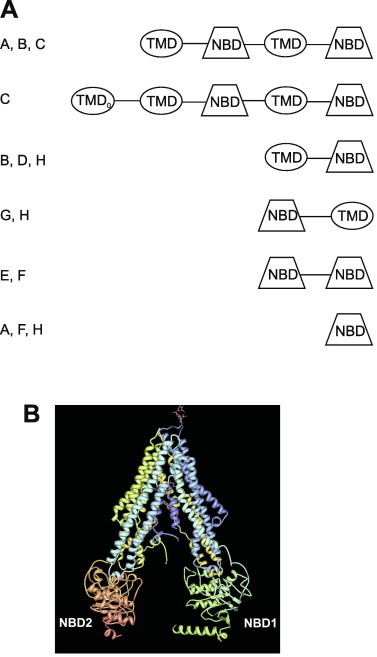
Structure of ABC multidrug transporters. (A) Predicted domain arrangement of ABC transporters. Shown are the arrangement of transmembrane domains (TMD) and nucleotide binding domains (NBD) found in ABC transporters. The TMD0 domain is found in some members of the ABCC sub-family. Letters on the left of the figure designate ABC sub-families in which that predicted domain topology is found. Figure adapted from (Sheps et al., 2004; Greenberg, 2013a). (B) Crystal structure of Pgp. Crystal structure of C. elegans Pgp (Jin et al., 2012; pdb 4F4C), as rendered in simple viewer (Moreland et al., 2005). NBD1 and NBD2 designate the nucleotide binding domains.
Pgp (ABCB1), the most thoroughly studied eukaryotic ABC multidrug transporter, is a glycosylated, ATP-dependent efflux transporter with broad substrate specificity. Its substrates comprise an extensive array of xenobiotics and other compounds, including many drugs; hence its important role in mediating drug resistance and MDR (Kartner et al., 1983). Reversal of MDR can be effected by members of a large and growing library of Pgp (and other ABC transporter) inhibitors. These compounds, many of which are drugs currently in clinical use, exhibit a wide range of potency and selectivity. Recently developed agents such as tariquidar and zosuquidar have been designed to target specific ABC transporters (e.g., Pgp), and exhibit enhanced selectivity and increased potency (Boumendjel et al., 2009; Morjani and Madoulet, 2009).
Pgp preferentially transports neutral and cationic hydrophobic compounds (Borst and Elferink, 2002; Ambudkar et al., 2003); other ABC transporters have substrate specificities that overlap somewhat with Pgp, but show important differences. For example, multidrug resistance associated protein 1 (MRP1; ABCC1) preferentially transports organic anions and Phase II metabolic products (e.g., glutathione-conjugates) likely to be found in the cytoplasm (Szakacs et al., 2006; Gimenez-Bonafe et al., 2008). As discussed above, Pgp and other ABC transporters can also translocate important signaling molecules such as glycolipids and phospholipids across the bilayer (Bosch et al., 1997; Romsicki and Sharom, 2001; Pohl et al., 2002; Mizutani et al., 2008; Aye et al., 2009). Indeed, possibly one of the most important physiological functions of ABC transporters may be to generate, maintain, and regulate membrane lipid asymmetry (Daleke, 2007; Sharom, 2011a). Eukaryotic ABC transporters typically act as ATP-dependent floppases, lipid transporters that translocate lipids away from the cytoplasmic face to the opposite (external) side of the membrane (flippases move lipids towards the cytoplasmic side of the bilayer; Fig 2).
Fig. 2.
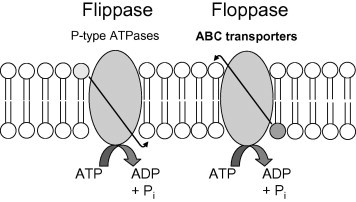
Model of ATP-dependent flippases and floppases. Flippases translocate lipids (typically phosphatidylserine and phosphatidylethanolamine) against a concentration gradient, towards the cytoplasmic face of the membrane. Floppases, exemplified by ABC transporters, translocate substrates (e.g., phosphatidylcholine, sphingolipids, cholesterol) in the opposite direction. Scramblases (not shown) are ATP-independent and calcium-dependent and transport lipids in both directions, along the concentration gradient, disrupting membrane asymmetry (Daleke, 2007; Sharom, 2011a).
Based on several lines of evidence, including the crystal structures of both mammalian (Aller et al., 2009; Li et al., 2014) and Caenorhabditis elegans (Jin et al., 2012) Pgp (Fig. 1B), the “hydrophobic vacuum cleaner” model is thought by most researchers to be a primary mechanism by which compounds interact with the binding pocket of Pgp. This model posits that Pgp “captures” these substrates while they are within the inner leaflet of the lipid bilayer (although access from the cytoplasm is also possible). Upon binding of ATP, Pgp either pumps or flips these substrates to the outer leaflet of the membrane or to the extracellular medium (Higgins and Gottesman, 1992; Sharom, 2011b). Jin et al. (2012) recently provided evidence in support of this model, finding that Pgp substrates within the membrane had up to a 4000-fold higher affinity for Pgp than those in detergent.
4. Physiological functions of ABC transporters
The critical association of ABC transporters with drug resistance and MDR has been a major driving force for much of the research on these proteins. As has been pointed out however, these transporters did not evolve to protect cancer cells and pathogens from medical interventions; instead, their ability to exclude toxins and xenobiotics from cells and tissues is likely co-opted and selected by drug treatment (Sarkadi et al., 2006). The most likely primary physiological role of these transporters is to act as a cellular defense system to protect cells and tissues from toxic agents. Interestingly, Sarkadi et al. (2006) have suggested that ABC transporters are part of a “chemoimmunity” network with innate and adaptive phases and features reminiscent of classical immunology. The adaptive phase is postulated to rely on a type of “memory” that depends on changes in transporter expression, efficiency, and coupling to other transporters and the general metabolic milieu, all of which are altered following an encounter with a particular toxin.
One manifestation of this protective function that has huge physiological implications occurs in vital tissues and organs requiring special protection (e.g., the brain), and is exemplified by the blood–brain barrier (BBB). ABC transporters such as Pgp and BCRP (ABCG2) serve as part of the mechanism in the BBB that blocks entry of potentially toxic compounds into the central nervous system (CNS) (Borst and Elferink, 2002). This barrier function of Pgp is highly relevant to at least one anthelmintic, the anti-nematodal drug ivermectin. The main target for ivermectin is the invertebrate-specific glutamate-gated chloride channel (Cleland, 1996; Wolstenholme, 2011); schistosomes also contain these channels, but they appear to be insensitive to ivermectin (Dufour et al., 2013)]. Part of the excellent safety profile of ivermectin also results from the fact that it is a substrate for mammalian Pgp, which mediates its exclusion from the host CNS. Loss or disruption of this Pgp function in the mammalian host can lead to ivermectin-induced neurological toxicity (Schinkel et al., 1994; Mealey et al., 2001). PZQ also has an outstanding safety profile, perhaps reflecting selective affinity for a platyhelminth-specific target (Greenberg, 2005b; Chan et al., 2012). However, there have been sporadic reports that PZQ can also affect molluscan and mammalian cells (Chubb et al., 1978; Gardner and Brezden, 1984). We have speculated (Greenberg, 2013a) that perhaps PZQ exclusion from the CNS (via mechanisms similar to those seen for ivermectin) is responsible in part for the excellent safety profile of the drug. On the other hand, PZQ has been reported not to be a substrate for mammalian Pgp (though it is an inhibitor; Hayeshi et al., 2006); one might speculate that a different ABC transporter such as BCRP is used to exclude PZQ from the host CNS. We are, however, unaware of any studies that test whether PZQ can act as a substrate for mammalian BCRP.
Several lines of evidence suggest that ABC transporters also play critical roles in normal cellular and organismal biology beyond this predominant protective activity (reviewed by Johnstone et al., 2000a; Mizutani et al., 2008). These include, among others: regulation of cell differentiation, proliferation, and survival, including apoptosis (Johnstone et al., 2000b); tumor promotion (Fletcher et al., 2010); and modulation of immune function (Mizutani et al., 2008; van de Ven et al., 2009a,b; Seyffir and Tampe, 2014). Interestingly, PZQ has been shown to synergistically enhance the efficacy of paclitaxel, a Pgp substrate, in inhibiting cancer cell growth, markedly decreasing expression of an anti-apoptotic protein (Wu et al., 2012). Additionally, mutations in ABCA transporters are associated with several disease states and members of this sub-family have been implicated in neurodegenerative disorders such as Alzheimer’s Disease (reviewed in van Meer et al., 2006; Kim et al., 2008; Piehler et al., 2012). Furthermore, as summarized in Table 1 and discussed above, many known or potential signaling molecules can serve as high-affinity substrates of different ABC transporters (Borst et al., 2000; Pohl et al., 2002; Sundaram et al., 2006; Mizutani et al., 2008; Aye et al., 2009; Quazi and Molday, 2011). As has been pointed out however (Borst et al., 1998; Borst and Elferink, 2002), some caution is in order when interpreting activities found under artificial experimental conditions (e.g., transporter inhibitors with variable selectivity and potency, gross overexpression of transporter proteins) as being reflective of genuine functions within the organism. Newer molecular genetic approaches may ultimately resolve some of this ambiguity.
5. ABC transporters in schistosomes
5.1. Drug susceptibility
We and others have recently reviewed the evidence that ABC transporters can influence drug susceptibility and may be associated with development and maintenance of drug resistance in schistosomes and other parasitic helminths (Kerboeuf et al., 2003; James et al., 2009a; Kasinathan and Greenberg, 2012; Lespine et al., 2012; Ardelli, 2013; Greenberg, 2013a). The association between helminth ABC transporters (most typically Pgp) and levels of anthelmintic susceptibility (and insusceptibility) is becoming more apparent, though a clear-cut, direct causal link between changes in transporter structure or expression and drug resistance remains elusive. However, we have shown that PZQ is an inhibitor and likely substrate of recombinant S. mansoni Pgp (Kasinathan et al., 2010), suggesting a mechanism by which disruption of Pgp function might increase effective PZQ concentration. Indeed, preliminary evidence from our group indicates that disruption of S. mansoni ABC transporter expression or function can potentiate the antischistosomal effects of PZQ (Greenberg et al., 2013). Pgp also appears to be associated with triclabendazole (TCBZ) resistance in another trematode, the liver fluke Fasciola hepatica (Wilkinson et al., 2012), and the Pgp inhibitor dexverapamil can enhance TCBZ activity in vitro against a TCBZ-resistant F. hepatica isolate (Savage et al., 2013). Similarly, inhibition of Pgp can enhance sensitivity of the nematode C. elegans to ivermectin (Ardelli and Prichard, 2013). These types of studies could potentially lead to new therapeutic strategies that combine anthelmintics with currently-approved drugs that, in addition to acting on their primary targets, block Pgp or other ABC transporters. If successful, such strategies could improve drug efficacy and possibly prevent emergence of drug resistance.
5.2. Excretory activity
Initial inquiries into genuine physiological functions of ABC transporters in schistosomes began over a decade ago, using fluorescent substrates of Pgp and MRP to visualize the parasite excretory system. The putative Pgp substrate resorufin exhibited energy-dependent concentration of fluorescence in the excretory tubules of adult worms (but not schistosomules), and allowed visualization of excretory activity via the nephridopore (Sato et al., 2002). Pgp inhibitors disrupted this excretory activity. Subsequent experiments using fluorescent MRP substrates showed similar results (Sato et al., 2004). Notably, PZQ (as well as other agents) disrupts the localization of resorufin, most potently in males (Kusel et al., 2006, 2009). Remarkably, an experimentally-induced PZQ-resistant isolate of schistosomes (LE-PZQ) is refractory to these effects of PZQ on resorufin labeling of the excretory system (Couto et al., 2010). As has been pointed out (Kusel et al., 2009), the excretory system of schistosomes is essential to the parasite’s survival, and ABC transporters that function within it offer candidate targets for disruption of vital parasite functions such as metabolic regulation, excretion of drugs and other toxic compounds, and interaction with the host.
5.3. Reproduction
As discussed in a recent review (Greenberg, 2013a), a 2003 patent showed that the calcium channel blockers verapamil and nifedipine significantly reduced egg production in both S. mansoni and the intestinal fluke Echinostoma caproni (Walter and Kuris, 2003). In addition to their activity against calcium channels, however, both of these drugs are relatively potent inhibitors of mammalian (Cornwell et al., 1987; Safa, 1988; Yang et al., 1988) and S. mansoni Pgp (Kasinathan et al., 2010). Using a combination of molecular genetic and pharmacological approaches, we confirmed the reported results on egg production in S. mansoni, and showed that these effects were attributable to interference with schistosome ABC transporters (Kasinathan et al., 2011). Furthermore, within the infected mouse host, Pgp and MRP1 inhibitors significantly reduced liver egg burden and pathology (Kasinathan et al., 2011).
The role ABC transporters might be playing in schistosome egg production is not yet clear. Eggs produced by worms exposed to ABC transporter inhibitors were often morphologically abnormal. However, isolated mature eggs exposed to these drugs appeared to be viable and hatched normally, suggesting that the transporter inhibitors are affecting egg development rather than the mature eggs directly. Limited analysis of the schistosome reproductive system using confocal microscopy suggested that the defect mapped primarily to females, and appeared to be manifested as a loss of immature oocytes and “piling up” of mature oocytes. Consistent with a female-specific effect, females exposed to transporter inhibitors could not be rescued by untreated males; they continued to exhibit reduced egg production (Kasinathan et al., 2011). Nonetheless, we have observed some limited evidence for a role of ABC transporters in male reproductive development as well. Clearly, a more rigorous and thorough examination of the role of ABC transporters in schistosome egg production is warranted, and could lead to new insights into development of the parasite reproductive system. It will also be important to determine whether these effects on egg production reflect a direct role for ABC transporters in this function or are rather a “collateral damage” due to interference with the activity of other organ systems dependent on these transporters, such as the gut or the excretory system (interestingly, such a situation may be occurring with ivermectin, which, as discussed below, interferes with excretory activity of filarial worms and suppresses production of microfilariae). Regardless of mechanism, however, as we have pointed out (Kasinathan et al., 2011; Greenberg, 2013a), these results could have practical implications for disease treatment and control strategies. Schistosome eggs cause the majority of pathology in schistosomiasis and they are also the agents of disease transmission. Use of agents that decrease parasite egg production, alone or in combination with anthelmintics, could be important in limiting host pathology and spread of drug resistance.
5.4. Parasite–host interactions
Several studies have implicated mammalian ABC transporters in influencing immune function (Mizutani et al., 2008; van de Ven et al., 2008, 2009a). ABC transporters have been linked to immunological functions such as Th1 skewing and activation (Pendse et al., 2006; Kooij et al., 2009), T cell migration (Randolph et al., 1998; Honig et al., 2003), and DC maturation and migration (Randolph et al., 1998; van de Ven et al., 2008; Kooij et al., 2009; van de Ven et al., 2009b). As discussed above, ABC transporters can translocate a wide array of compounds with biological signaling capability (Table 1), and in some cases, transporters and their substrates have been linked directly to functional outputs. TAP1 and TAP2, heterodimeric ABC transporters (ABCB2 + ABCB3), deliver cytosolic peptides to class I MHC molecules for antigen presentation (Koopmann et al., 1996; McCluskey et al., 2004; Hinz and Tampe, 2012; Seyffir and Tampe, 2014). ABC transporters have also been implicated in pro-inflammatory cytokine efflux (Drach et al., 1996; Raghu et al., 1996; Frank et al., 2001; Pawlik et al., 2005; Pendse et al., 2006), though mice lacking mdr1a-encoded Pgp exhibit relatively normal cytokine levels and T-cell function (Eisenbraun and Miller, 1999). Others have suggested that ABC transporters may influence cytokine production indirectly, through bioactive lipid compounds that are substrates of ABC transporters (Kooij et al., 2012). There is also evidence that cytokines themselves can influence ABC transporter expression in immune cells (Liptrott et al., 2009).
Schistosomes and other parasites are hugely successful at, and indeed depend for their survival and development on, modulating and manipulating host immune responses (Pearce and Macdonald, 2002; Lamb et al., 2010). How the host immune system responds to the parasite determines in large part the balance between protective immunity (health) and immunopathology (morbidity) (Wilson et al., 2007). Interestingly, others have suggested that ivermectin interference with the microfilarial excretory–secretory apparatus in filarial worms might be altering parasite modulation of host responses, contributing to the rapid clearance of microfilariae from the host (Moreno et al., 2010). Could schistosomes and other parasites use their ABC transporters as part of the apparatus by which they influence host responses?
Substrates of ABC transporters with potential immune signaling activity (see Table 1) include compounds implicated in schistosome modulation of host responses such as glycolipids (Van der Kleij et al., 2002b), lipids and phospholipids (e.g., phosphatidylserine and lyso-phosphatidylserine; van der Kleij et al., 2002a; van Riet et al., 2009), and dsRNAs (Aksoy et al., 2005). Excretory/secretory products of schistosomules and adults contain classes of immunomodulators such as eicosanoids (Salafsky and Fusco, 1987; Angeli et al., 2001) that can serve as high-affinity ABC transporter substrates. Furthermore, schistosomule-derived prostaglandin D2 inhibits migration of epidermal Langerhans cells, dendritic cells that play a key role in establishing cutaneous immunity, to the skin-draining lymph nodes (Angeli et al., 2001). Interestingly, in our studies on the role of schistosome ABC transporters in egg production, we found that S. mansoni-infected mice administered Pgp inhibitors showed not just a reduction in liver granuloma number (corresponding to the lower egg burden), but also a significant decrease in granuloma size (Kasinathan et al., 2011). Such a difference in granuloma size is not predicted or explained by the reduction in egg burden, and could indicate an altered host immune response to the parasites, perhaps reflecting an effect of the Pgp inhibitors on the immunomodulatory properties of the eggs (or, of course, on host cells, or on both).
These observations suggest that schistosome (or other helminth) ABC transporters may have been co-opted by parasites to constitute part of the mechanism used to shape and manipulate host responses. Clearly, this question is worth pursuing, as it could provide information on novel roles for this class of transporters as well as possible new therapies that interfere with this function. By framing the questions correctly, the combination of the rich pharmacology of these transporters with the availability of powerful molecular genetic approaches should allow researchers to dissect the role of ABC transporters in these complex interactions, and could provide important insights into the mechanisms underlying parasite–host interactions.
6. Conclusions
This review has outlined two parallel inquiries regarding the role of ABC transporters in schistosomes. First, there is increasing evidence that schistosome (and other helminth) ABC transporters can modulate PZQ sensitivity. If that is indeed the case, it may provide the impetus to test repurposed ABC transporter inhibitors in combination therapy to potentiate PZQ activity. This prospect is exciting, as it could increase the real-world effectiveness of PZQ, particularly against immature worms, as well as prevent emergence or spread of resistance. As we have discussed previously (Kasinathan and Greenberg, 2012; Greenberg, 2013a), such an approach would likely involve only one or two doses in combination with standard PZQ treatment, thereby reducing the likelihood of encountering the types of complications seen with long-term, high-dose regimens tested in clinical trials to enhance cancer chemotherapy (Szakacs et al., 2006; Shukla et al., 2008; Coley, 2010).
The second line of inquiry focuses on the genuine functions of these transporters in the parasite. Though current work has barely scratched the surface of this topic, it seems obvious that ABC transporters are likely to be quite important for schistosome survival and development within its two very different hosts, if only to keep toxins and xenobiotics in check. Given the limited number of studies focused on these proteins in parasites, it is quite striking that they have already been implicated in schistosome excretory activity and reproduction, two functions that are vital to parasite survival, transmission, and pathogenicity. The possibility that schistosome ABC transporters are involved in presentation of parasite immunomodulatory factors to the host is intriguing, and if validated, suggests yet another novel function for members of this ancient and highly diverse protein superfamily.
Conflict of interest
The author declared that there is no conflict of interest.
Acknowledgments
R.M.G. is supported by NIH Grants R21AI112713, R21AI106268, and R21AI100505 and by a Bill & Melinda Gates Foundation Grand Challenges Explorations award.
References
- Aksoy E., Zouain C.S., Vanhoutte F., Fontaine J., Pavelka N., Thieblemont N., Willems F., Ricciardi-Castagnoli P., Goldman M., Capron M., Ryffel B., Trottein F. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 2005;280:277–283. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- Aller S.G., Yu J., Ward A., Weng Y., Chittaboina S., Zhuo R., Harrell P.M., Trinh Y.T., Zhang Q., Urbatsch I.L., Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar S.V., Kimchi-Sarfaty C., Sauna Z.E., Gottesman M.M. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- Andrews P., Thomas H., Pohlke R., Seubert J. Praziquantel. Med. Res. Rev. 1983;3:147–200. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- Angeli V., Faveeuw C., Rove O., Fontaine J., Teissiere E., Capron A., Wolowczuk I., Capron M., Trottein F. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 2001;193:1135–1147. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annilo T., Chen Z.Q., Shulenin S., Constantino J., Thomas L., Lou H., Stefanov S., Dean M. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics. 2006;88:1–11. doi: 10.1016/j.ygeno.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Aragon A.D., Imani R.A., Blackburn V.R., Cupit P.M., Melman S.D., Goronga T., Webb T., Loker E.S., Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelli B.F. Transport proteins of the ABC systems superfamily and their role in drug action and resistance in nematodes. Parasitol. Int. 2013;62:639–646. doi: 10.1016/j.parint.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Ardelli B.F., Prichard R.K. Inhibition of P-glycoprotein enhances sensitivity of Caenorhabditis elegans to ivermectin. Vet. Parasitol. 2013;191:264–275. doi: 10.1016/j.vetpar.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Aye I.L.M.H., Singh A.T., Keelan J.A. Transport of lipids by ABC proteins: interactions and implications for cellular toxicity, viability, and function. Chem. Biol. Interact. 2009;180:327–339. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Borst P., Elferink R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Borst P., van Blitterswijk W.J., Borst J., Tepper A.D., Schinkel A.H. New physiological functions for drug-transporting P-glycoproteins? Drug Resist. Updates. 1998;1:337–339. doi: 10.1016/s1368-7646(98)80049-6. [DOI] [PubMed] [Google Scholar]
- Borst P., Zelcer N., van Helvoort A. ABC transporters in lipid transport. Biochim. Biophys. Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- Bosch I., Dunussi-Joannopoulos K., Wu R.L., Furlong S.T., Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–5694. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- Boumendjel A., Florin A., Boutonnat J. Reversal agents of multidrug resistance mediated by multidrug resistance-associated proteins (MRPs) In: Boumendjel A., Boutonnat J., Robert J., editors. ABC Transporters and Multidrug Resistance. John Wiley & Sons Inc; Hoboken, NJ: 2009. pp. 261–288. [Google Scholar]
- Caffrey C.R. Chemotherapy of schistosomiasis: present and future. Curr. Opin. Chem. Biol. 2007;11:433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Chai J.-Y. Praziquantel treatment in trematode and cestode infections: an update. Infection and Chemotherapy. 2013;45:32–43. doi: 10.3947/ic.2013.45.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., Agbedanu P.N., Zamamian M., Gruba S.M., Haynes C.L., Day T.A., Marchant J.S. ‘Death and axes’: unexpected Ca2+ entry phenologs predict new anti-schistosomal agents. PLoS Pathog. 2014;10:e1003942. doi: 10.1371/journal.ppat.1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., Zarowiecki M., Marchant J.S. Ca2+ channels and praziquantel: a view from the free world. Parasitol. Int. 2012 doi: 10.1016/j.parint.2012.12.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.S., Lee K., Kruh G.D. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J. Biol. Chem. 2001;276:33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- Chubb J.M., Bennett J.L., Akera T., Brody T.M. Effects of praziquantel, a new anthelmintic, on electromechanical properties of isolated rat atria. J. Pharmacol. Exp. Therapeutics. 1978;207:284–293. [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L. Praziquantel. Parasitol. Res. 2003;90(Suppl. 1):S3–S9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- Cioli D., Pica Mattoccia L., Basso A., Guidi A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 2014;21:21–25. doi: 10.1016/j.molbiopara.2014.06.002. in press. [DOI] [PubMed] [Google Scholar]
- Cleland T.A. Inhibitory glutamate receptor channels. Mol. Neurobiol. 1996;13:97–136. doi: 10.1007/BF02740637. [DOI] [PubMed] [Google Scholar]
- Coley H.M. Overcoming multidrug resistance in cancer: clinical studies of P-glycoprotein inhibitors. Methods Mol. Biol. 2010;596:341–358. doi: 10.1007/978-1-60761-416-6_15. [DOI] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell M.M., Pastan I., Gottesman M.M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J. Biol. Chem. 1987;262:2166–2170. [PubMed] [Google Scholar]
- Couto F.F., Coelho P.M., Araujo N., Kusel J.R., Katz N., Mattos A.C. Use of fluorescent probes as a useful tool to identify resistant Schistosoma mansoni isolates to praziquantel. Parasitology. 2010;137:1791–1797. doi: 10.1017/S003118201000065X. [DOI] [PubMed] [Google Scholar]
- Cui Y., Konig J., Buchholz J.K., Spring H., Leier I., Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- Daleke D.L. Phospholipid flippases. J. Biol. Chem. 2007;282:821–825. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- Dassa E., Bouige P. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/s0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- Day T.A., Botros S. Drug resistance in schistosomes. In: Maule A., Marks N.J., editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. CAB International; Oxfordshire, UK: 2006. pp. 256–268. [Google Scholar]
- Dean M., Allikmets R. Complete characterization of the human ABC gene family. J. Bioenerg. Biomembr. 2001;33:475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- Dean M., Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J., Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Exp. Rev. Anti-infect. Ther. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- Drach J., Gsur A., Hamilton G., Zhao S., Angerler J., Fiegl M., Zojer N., Raderer M., Haberl I., Andreeff M., Huber H. Involvement of P-glycoprotein in the transmembrane transport of interleukin-2 (IL-2), IL-4, and interferon-gamma in normal human T lymphocytes. Blood. 1996;88:1747–1754. [PubMed] [Google Scholar]
- Dufour V., Beech R.N., Wever C., Dent J.A., Geary T.G. Molecular cloning and characterization of novel glutamate-gated chloride channel subunits from Schistosoma mansoni. PLoS Pathog. 2013;9:e1003586. doi: 10.1371/journal.ppat.1003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbraun M.D., Miller R.A. Mdr1a-encoded P-glycoprotein is not required for peripheral T cell proliferation, cytokine release, or cytotoxic effector function in mice. J. Immunol. 1999;163:2621–2627. [PubMed] [Google Scholar]
- Ernest S., Bello-Reuss E. Secretion of platelet-activating factor is mediated by MDR1 P-glycoprotein in cultured human mesangial cells. J. Am. Soc. Nephrol. 1999;10:2306–2313. doi: 10.1681/ASN.V10112306. [DOI] [PubMed] [Google Scholar]
- Fletcher J.I., Haber M., Henderson M.J., Norris M.D. ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- Frank M.H., Denton M.D., Alexander S.I., Khoury S.J., Sayegh M.H., Briscoe D.M. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J. Immunol. 2001;166:2451–2459. doi: 10.4049/jimmunol.166.4.2451. [DOI] [PubMed] [Google Scholar]
- Garba A., Lamine M.S., Barkire N., Djibo A., Sofo B., Gouvras A.N., Labbo R., Sebangou H., Webster J.P., Fenwick A., Utzinger J. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop. 2013;128:334–344. doi: 10.1016/j.actatropica.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Gardner D.R., Brezden B.L. The sites of action of praziquantel in a smooth muscle of Lymnaea stagnalis. Can. J. Physiol. Pharmacol. 1984;62:282–287. doi: 10.1139/y84-044. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Woo K., McCarthy J.S., Mackenzie C.D., Horton J., Prichard R.K., de Silva N.R., Olliaro P.L., Lazdins-Helds J.K., Engels D.A., Bundy D.A. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int. J. Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gimenez-Bonafe P., Guillen Canovas A., Ambrosio S., Tortosa A., Perez-Tomas R. Drugs modulating MDR. In: Colabufo N.A., editor. Research Signpost. Kerala; India: 2008. pp. 63–99. [Google Scholar]
- Greenberg R.M. Are Ca2+ channels targets of praziquantel action? Int. J. Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Greenberg R.M. Ca2+ signalling, voltage-gated Ca2+ channels and praziquantel in flatworm neuromusculature. Parasitology. 2005;131(Suppl.):S97–S108. doi: 10.1017/S0031182005008346. [DOI] [PubMed] [Google Scholar]
- Greenberg R.M. ABC multidrug transporters in schistosomes and other parasitic flatworms. Parasitol. Int. 2013;62:647–653. doi: 10.1016/j.parint.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology. 2013;140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M., John B., Hunter C.A., Kasinathan R.S. Schistosome ABC multidrug transporters: roles in parasite physiology, drug susceptibility and immunomodulatory signaling. Am. J. Trop. Med. Hyg. 2013;89:A953. [Google Scholar]
- Guo Y., Kotova E., Chen Z.S., Lee K., Hopper-Borge E., Belinsky M.G., Kruh G.D. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J. Biol. Chem. 2003;278:29509–29514. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- Hayeshi R., Masimirembwa C., Mukanganyama S., Ungell A.L. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur. J. Pharm. Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Higgins C.F., Gottesman M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Hines-Kay J., Cupit P.M., Sanchez M.C., Rosenberg G.H., Hanelt B., Cunningham C. Transcriptional analysis of Schistosoma mansoni treated with praziquantel in vitro. Mol. Biochem. Parasitol. 2012;186:87–94. doi: 10.1016/j.molbiopara.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A., Tampe R. ABC transporters and immunity: mechanism of self-defense. Biochemistry. 2012;51:4981–4989. doi: 10.1021/bi300128f. [DOI] [PubMed] [Google Scholar]
- Honig S.M., Fu S., Mao X., Yopp A., Gunn M.D., Randolph G.J., Bromberg J.S. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J. Clin. Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Platzer E.G., Bellier A., Aroian R.V. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. Proc. Natl. Acad. Sci. 2010;107:5955–5960. doi: 10.1073/pnas.0912327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S.C., Emsley P., Hartshorn M.J., Mimmack M.M., Gileadi U., Pearce S.R., Gallagher M.P., Gill D.R., Hubbard R.E., Higgins C.F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- James C.E., Hudson A.L., Davey M.W. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol. 2009;25:328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- James C.E., Hudson A.L., Davey M.W. An update on P-glycoprotein and drug resistance in Schistosoma mansoni. Trends Parasitol. 2009;25:538–539. doi: 10.1016/j.pt.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Jin M.S., Oldham M.L., Zhang Q., Chen J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 2012;490:566–569. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R.W., Ruefli A.A., Smyth M.J. Multiple physiological functions for multidrug transporter P-glycoprotein? Trends Biochem. Sci. 2000;25:1–6. doi: 10.1016/s0968-0004(99)01493-0. [DOI] [PubMed] [Google Scholar]
- Johnstone R.W., Ruefli A.A., Tainton K.M., Smyth M.J. A role for P-glycoprotein in regulating cell death. Leukemia and Lymphoma. 2000;38:1–11. doi: 10.3109/10428190009060314. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J.R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kasinathan R.S., Greenberg R.M. Pharmacology and potential physiological significance of schistosome multidrug resistance transporters. Exp. Parasitol. 2012;132:2–6. doi: 10.1016/j.exppara.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinathan R.S., Goronga T., Messerli S.M., Webb T.R., Greenberg R.M. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB J. 2010;24:128–135. doi: 10.1096/fj.09-137091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinathan R.S., Morgan W.M., Greenberg R.M. Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2011;5:e1425. doi: 10.1371/journal.pntd.0001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerboeuf D., Blackhall W., Kaminsky R., von Samson-Himmelstjerna G. P-glycoprotein in helminths: function and perspectives for anthelmintic treatment and reversal of resistance. Int. J. Antimicrob. Agents. 2003;22:332–346. doi: 10.1016/s0924-8579(03)00221-8. [DOI] [PubMed] [Google Scholar]
- Kim W.S., Weickert C.S., Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J. Neurochem. 2008;104:1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illness. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- King C.H., Olbrych S.K., Soon M., Singer M.E., Carter J., Colley D.G. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl. Trop. Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij G., Backer R., Koning J.J., Reijerkerk A., van Horssen J., van der Pol S.M.A., Drexhage J., Schinkel A., Dijkstra C.D., den Haan J.M.M., Geijtenbeek T.B.H., de Vries H.E. P-Glycoprotein Acts as an Immunomodulator during Neuroinflammation. PLoS ONE. 2009;4:e8212. doi: 10.1371/journal.pone.0008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij G., van Horssen J., Bandaru W., Haughey N.J., De Vries H.E. The role of ATP-binding cassette transporters in neuro-inflammation: relevance for bioactive lipids. Front. Pharmacol. 2012;3:74. doi: 10.3389/fphar.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann J.O., Post M., Neefjes J.J., Hammerling G.J., Momburg F. Translocation of long peptides by transporters associated with antigen processing (TAP) Eur. J. Immunol. 1996;26:1720–1728. doi: 10.1002/eji.1830260809. [DOI] [PubMed] [Google Scholar]
- Kusel J.R., McVeigh P., Thornhill J.A. The schistosome excretory system: a key to regulation of metabolism, drug excretion and host interaction. Trends Parasitol. 2009;25:353–358. doi: 10.1016/j.pt.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Kusel J.R., Oliveira F.A., Todd M., Ronketti F., Lima S.F., Mattos A.C., Reis K.T., Coelho P.M., Thornhill J.A., Ribeiro F. The effects of drugs, ions, and poly-l-lysine on the excretory system of Schistosoma mansoni. Mem. Inst. Oswaldo Cruz. 2006;101:293–298. doi: 10.1590/s0074-02762006000900046. [DOI] [PubMed] [Google Scholar]
- Lamb E.W., Walls C.D., Pesce J.T., Riner D.K., Maynard S.K., Crow E.T., Wynn T.A., Schaefer B.C., Davies S.J. Blood fluke exploitation of non-cognate CD4+ T cell help to facilitate parasite development. PLoS Pathog. 2010;29:e1000892. doi: 10.1371/journal.ppat.1000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buccholz U., Cole S.P., Deeley R.G., Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994;269:27807–27810. [PubMed] [Google Scholar]
- Leprohon P., Legare D., Ouellette M. ABC transporters involved in drug resistance in human parasites. Essays Biochem. 2011;50:121–144. doi: 10.1042/bse0500121. [DOI] [PubMed] [Google Scholar]
- Lespine A., Alvinerie M., Vercruysse J., Prichard R.K., Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Lespine A., Menez C., Bourguinat C., Prichard R.K. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: prospects for reversing transport-dependent anthelmintic resistance. Int. J. Parasitol.: Drugs Drug Resistance. 2012;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Jaimes K.F., Aller S.G. Refined structures of mouse P-glycoprotein. Protein Sci. 2014;23:34–46. doi: 10.1002/pro.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.J., Aszalos A. Multidrug transporters as drug targets. Curr. Drug Targets. 2006;7:911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- Liptrott N.J., Penny M., Bray P.G., Sathish J., Khoo S.H., Back D.J., Owen A. The impact of cytokines on the expression of drug transporters, cytochrome P450 enzymes and chemokine receptors in human PBMC. Br. J. Pharmacol. 2009;156:497–508. doi: 10.1111/j.1476-5381.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey J., Rossjohn J., Purcell A.W. TAP genes and immunity. Curr. Opin. Immunol. 2004;16:651–659. doi: 10.1016/j.coi.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Mealey K.L., Bentjen S.A., Gay J.M., Cantor G.H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11:727–733. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Masuda M., Nakai E., Furumiya K., Togawa H., Nakamura Y., Kawai Y., Nakahira K., Shinkai S., Takahashi K. Genuine functions of P-glycoprotein (ABCB1) Curr. Drug Metab. 2008;9:167–174. doi: 10.2174/138920008783571756. [DOI] [PubMed] [Google Scholar]
- Moreland J.L., Gramada A., Buzko O.V., Zhang Q., Bourne P.E. The molecular biology toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinformatics. 2005;6:21. doi: 10.1186/1471-2105-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morjani H., Madoulet C. Reversal agents for P-glycoprotein-mediated multidrug resistance. In: Boumendjel A., Boutonnat J., Robert J., editors. ABC Transporters and Multidrug Resistance. John Wiley & Sons Inc; Hoboken, NJ: 2009. pp. 241–259. [Google Scholar]
- Mutapi F., Rujeni N., Bourke C., Mitchell K., Appleby L., Nausch N., Midzi N., Mduluza T. Schistosoma haematobium treatment in 1–5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl. Trop. Dis. 2011;5:e1143. doi: 10.1371/journal.pntd.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeffo Mbah M.L., Poolman E.M., Atkins K.E., Orenstein E.W., Meyers L.A., Townsend J.P., Galvani A.P. Potential cost-effectiveness of schistosomiasis treatment for reducing HIV transmission in Africa – the case of Zimbabwean women. PLoS Negl. Trop. Dis. 2013;7:e2346. doi: 10.1371/journal.pntd.0002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik A., Baskiewicz-Masiuk M., Machalinski B., Kurzawski M., Gawronska-Szklarz B. Involvement of C3435T and G2677T multidrug resistance gene polymorphisms in release of cytokines from peripheral blood mononuclear cells treated with methotrexate and dexamethasone. Eur. J. Pharmacol. 2005;528:27–36. doi: 10.1016/j.ejphar.2005.10.068. [DOI] [PubMed] [Google Scholar]
- Pearce E.J., Macdonald C.A. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Pendse S.S., Behjadi S., Schatton T., Izawa A., Sayegh M.H., Frank M.H. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am. J. Transplant. 2006;6:2884–2893. doi: 10.1111/j.1600-6143.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Orsini T., Basso A., Festucci A., Liberti P., Guidi A., Marcatto-Maggi A.L., Nobre-Santana S., Troiani A.R., Cioli D., Valle C. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp. Parasitol. 2008;119:332–335. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Piehler A.P., Ozcurumez M., Kaminski W.E. A-subclass ATP-binding cassette proteins in brain lipid homeostasis and neurodegeneration. Front. Psychiatry. 2012;3:17. doi: 10.3389/fpsyt.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl A., Lage H., Muller P., Pomorski T., Herrmann A. Transport of phosphatidylserine via MDR1 (multidrug resistance 1) P-glycoprotein in a human gastric carcinoma cell line. Biochem. J. 2002;365:259–268. doi: 10.1042/BJ20011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quazi F., Molday R.S. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011;50:265–290. doi: 10.1042/bse0500265. [DOI] [PubMed] [Google Scholar]
- Raggers R.J., Vogels I., van Meer G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem. J. 2001;357:859–865. doi: 10.1042/0264-6021:3570859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G., Park S.W., Roninson I.B., Mechetner E.B. Monoclonal antibodies against P-glycoprotein, an MDR1 gene product, inhibit interleukin-2 release from PHA-activated lymphocytes. Exp. Hematol. 1996;24:1258–1264. [PubMed] [Google Scholar]
- Randolph G.J., Beaulieu S., Pope M., Sugawara I., Hoffman L., Steinman R.M., Muller W.A. A physiologic function for P-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. 1998;95:6924–6929. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C.A., Robertson A., Fallon P.G., Modha J., Kusel J.R., Doenhoff M.J., Martin R.J. Praziquantel: an urgent and exciting challenge. Parasitol. Today. 1996;12:14–20. doi: 10.1016/0169-4758(96)80640-5. [DOI] [PubMed] [Google Scholar]
- Reid G., Wielinga P., Zelcer N., van der Heijden J.W., Kuil A., de Haas M., Wijnholds J., Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad Sci. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius M., Hummel-Eisenbeiss J., Keppler D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4) J. Phamacol. Exp. Ther. 2008;324:86094. doi: 10.1124/jpet.107.131342. [DOI] [PubMed] [Google Scholar]
- Robb S.M., Ross E., Sanchez Alvarado A. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:599–606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romsicki Y., Sharom F.J. Phospholipid flippase activity of the reconstituted P-glycoprotein multidrug transporter. Biochemistry. 2001;40:6937–6947. doi: 10.1021/bi0024456. [DOI] [PubMed] [Google Scholar]
- Sabah A.A., Fletcher C., Webbe G., Doenhoff M.J. Schistosoma mansoni: chemotherapy of infections of different ages. Exp. Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- Safa A.R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc. Natl. Acad. Sci. 1988;85:187–191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M.H., Paulsen I.T. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 2001;12:205–213. doi: 10.1006/scdb.2000.0246. [DOI] [PubMed] [Google Scholar]
- Salafsky B., Fusco A.C. Schistosoma mansoni: a comparison of secreted vs nonsecreted eicosanoids in developing schistosomulae and adults. Exp. Parasitol. 1987;64:361–367. doi: 10.1016/0014-4894(87)90048-8. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Homolya L., Szakacs G., Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol. Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- Sato H., Kusel J.R., Thornhill J. Functional visualization of the excretory system of adult Schistosoma mansoni by the fluorescent marker resorufin. Parasitology. 2002;125:527–535. doi: 10.1017/s0031182002002536. [DOI] [PubMed] [Google Scholar]
- Sato H., Kusel J.R., Thornhill J. Excretion of fluorescent substrates of mammalian multidrug resistance-associated protein (MRP) in the Schistosoma mansoni excretory system. Parasitology. 2004;128:43–52. doi: 10.1017/s0031182003004177. [DOI] [PubMed] [Google Scholar]
- Savage J., Meaney M., Brennan G.P., Hoey E., Trudgett A., Fairweather I. Increased action of triclabendazole (TCBZ) in vitro against a TCBZ-resistant isolate of Fasciola hepatica following its co-incubation with the P-glycoprotein inhibitor, R(+)-verapamil. Exp. Parasitol. 2013;135:642–653. doi: 10.1016/j.exppara.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Schinkel A.H., Smit J.J., van Tellingen O., Beijnen J.H., Wagenaar E., van Deemter L., Mol C.A., van der Valk M.A., Robanus-Maandag E.C., te Riele H.P., Berns A.J.M., Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Sesay S., Paye J., Bah M.S., McCarthy F.M., Conteh A., Sonnie M., Hodges M.H., Zhang Y. Schistosoma mansoni infection after three years of mass drug administration in Sierra Leone. Parasite Vector. 2014;7:14. doi: 10.1186/1756-3305-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyffir F., Tampe R. ABC transporters in adaptive immunity. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbagen.2014.05.022. in press. [DOI] [PubMed] [Google Scholar]
- Sharom F.J. Flipping and flopping–lipids on the move. IUBMB Life. 2011;63:736–746. doi: 10.1002/iub.515. [DOI] [PubMed] [Google Scholar]
- Sharom F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–178. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- Sheps J.A., Ralph S., Zhao Z.Y., Baillie D.L., Ling V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 2004;5:R15. doi: 10.1186/gb-2004-5-3-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Wu C.P., Ambudkar S.V. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Exp. Opin. Drug Metabol. Toxicol. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- Sousa-Figueiredo J.C., Betson M., Atuhaire A., Arinaitwe M., Navaratnam A.M., Kabatereine N.B., Bickle Q., Stothard J.R. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl. Trop. Dis. 2012;6:e1864. doi: 10.1371/journal.pntd.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock R.F. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem. Inst. Oswaldo Cruz. 2001;96(Supplement):17–27. doi: 10.1590/s0074-02762001000900003. [DOI] [PubMed] [Google Scholar]
- Sundaram P., Echalier B., Han W., Hull D., Timmons L. ATP-binding cassette transporters are required for efficient RNA interference in Caenorhabditis elegans. Mol. Biol. Cell. 2006;17:3678–3688. doi: 10.1091/mbc.E06-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakacs G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Tsuji M. From discovery to eradication of schistosomiasis in Japan: 1847–1996. Int. J. Parasitol. 1997;27:1465–1480. doi: 10.1016/s0020-7519(97)00183-5. [DOI] [PubMed] [Google Scholar]
- Toure S., Zhang Y., Bosque-Oliva E., Ky C., Ouedraogo A., Koukounari A., Gabrielli A.F., Bertrand S., Webster J.P., Fenwick A. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bull. World Health Organ. 2008;86:780–787. doi: 10.2471/BLT.07.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukahebwa E., Vennervald B.J., Nuwaha F., Kabatereine N.B., Magnussen P. Comparative efficacy of one versus two doses of praziquantel on cure rate of Schistosoma mansoni infection and re-infection in Mayuge District, Uganda. Trans. R. Soc. Trop. Med. Hyg. 2013;107:397–404. doi: 10.1093/trstmh/trt024. [DOI] [PubMed] [Google Scholar]
- van de Ven R., Scheffer G.L., Reurs A.W., Lindenberg J.J., Oerlemans R., Jansen G., Gillet J.P., Glasgow J.N., Pereboev A., Curiel D.T., Scheper R.J., de Gruijl T.D. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood. 2008;112:2353–2359. doi: 10.1182/blood-2008-03-147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven R., Oerlemans R., van der Heijden J.W., Scheffer G.L., de Gruijl T.D., Jansen G., Scheper R.J. ABC drug transporters and immunity: novel therapeutic targets in autoimmunity and cancer. J. Leukoc. Biol. 2009;86:1075–1087. doi: 10.1189/jlb.0309147. [DOI] [PubMed] [Google Scholar]
- van de Ven R., Scheffer G.L., Scheper R.J., de Gruijl T.D. The ABC of dendritic cell development and function. Trends Immunol. 2009;30:421–429. doi: 10.1016/j.it.2009.06.004. [DOI] [PubMed] [Google Scholar]
- van der Kleij D., Latz E., Brouwers J.F.H.M., Kruize Y.C.M., Schmitz M., Kurt-Jones E.A., Espevik T., de Jong E.C., Kapsenberg M.L., Golenbock D.T., Tielens A.G.M., Yazdanbakhsh M. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- Van der Kleij D., Van Remoortere A., Schuitemaker J.H., Kapsenberg M.L., Deeider A.M., Tielens A.G.M., Hokke C.H., Yazdanbakhsh M. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc beta 1–4(Fuc alpha 1–2Fuc alpha 1–3)GlcNAc. J. Infect. Dis. 2002;185:531–539. doi: 10.1086/338574. [DOI] [PubMed] [Google Scholar]
- van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- van Meer G., Halter D., Sprong H., Somerharju P., Egmond M.R. ABC lipid transporters: extruders, flippases, or flopless activators? FEBS Lett. 2006;580:1171–1177. doi: 10.1016/j.febslet.2005.12.019. [DOI] [PubMed] [Google Scholar]
- van Riet E., Everts B., Retra K., Phylipsen M., van Hellemond J.J., Tielens A.G.M., van der Kleij D., Hartgers F.C., Yazdanbakhsh M. Combined TLR2 and TLR4 ligation in the context of bacterial or helminth extracts in human monocyte derived dendritic cells: molecular correlates for Th1/Th2 polarization. BMC Immunol. 2009;10:9. doi: 10.1186/1471-2172-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennervald B.J., Booth M., Butterworth A.E., Kariuki H.C., Kadzo H., Ireri E., Amaganga C., Kimani G., Kenty L., Mwatha J., Ouma J.H., Dunne D.W. Regression of hepatosplenomegaly in Kenyan school-aged children after praziquantel treatment and three years of greatly reduced exposure to Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 2005;99:150–160. doi: 10.1016/j.trstmh.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Walter, M., Kuris, A., 2003. Methods for the inhibition of egg production in trematodes. US Patent Number 6,514,963 B2.
- Wang W., Wang L., Liang Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 2012;111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- Wielinga P.R., van der Heijden I., Reid G., Beijnen J.H., Wijnholds J., Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J. Biol. Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- Wilkinson R., Law C.J., Hoey E.M., Fairweather I., Brennan G.P., Trudgett A. An amino acid substitution in Fasciola hepatica P-glycoprotein from triclabendazole-resistant and triclabendazole-susceptible populations. Mol. Biochem. Parasitol. 2012;186:69–72. doi: 10.1016/j.molbiopara.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Mentink-Kane M.M., Pesce J.T., Ramalingam T.R., Thompson R., Wynn T.A. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A. Ion channels and receptor as targets for the control of parasitic nematodes. Int. J. Parasitol.: Drugs Drug Resistance. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.H., Lu M.K., Hu L.Y., Li X. Praziquantel synergistically enhances paclitaxel efficacy to inhibit cancer cell growth. PLoS ONE. 2012;7:e51721. doi: 10.1371/journal.pone.0051721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.H., Catto B.A., Webster L.T., Jr. Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J. Infect. Dis. 1985;151:1130–1137. doi: 10.1093/infdis/151.6.1130. [DOI] [PubMed] [Google Scholar]
- Yang C.P., Mellado W., Horwitz S.B. Azidopine photoaffinity labeling of multidrug resistance-associated glycoproteins. Biochem. Pharmacol. 1988;37:1417–1421. doi: 10.1016/0006-2952(88)90803-9. [DOI] [PubMed] [Google Scholar]


