Abstract
Background
The aims of this study were to determine hippuric acid levels in urine samples, airborne toluene levels, acute and chronic neurological symptoms, and to describe any correlation between urinary hippuric acid and airborne toluene.
Methods
The hippuric acid concentration in the urine of 87 paint workers exposed to toluene at work (exposed group), and 87 nonexposed people (control group) was studied. Study participants were selected from similar factories in the same region. Urine samples were collected at the end of a shift and analyzed for hippuric acid by high performance liquid chromatography. Air samples for the estimation of toluene exposure were collected with diffusive personal samplers and the toluene quantified using gas–liquid chromatography. The two groups were also interviewed and observed about their work practices and health.
Results
The median of the 87 airborne toluene levels was 55 ppm (range, 12–198 ppm). The median urinary hippuric acid level was 800 mg/g creatinine (range, 90–2547 mg/g creatinine). A statistically significant positive correlation was found between airborne toluene exposure and urine hippuric acid levels (r = 0.548, p < 0.01). Workers with acute symptoms had significantly higher hippuric acid levels than those who did not (p < 0.05). It was concluded that there was a significant correlation between toluene exposure, hippuric acid levels, and health (p < 0.001).
Conclusion
There appears to be a significant correlation between workers exposure to toluene at work, their urine hippuric acid levels, and resulting symptoms of poor health. Improvements in working conditions and occupational health education are required at these workplaces. There was good correlation between urinary hippuric acid and airborne toluene levels.
Keywords: health, hippuric acid, paint workers, steel furniture manufacture, toluene
1. Introduction
Toluene (methyl benzene) is a colorless, flammable, volatile liquid with a distinctive aroma. The largest users of “recovered” toluene are companies that make benzene. In 2008–2009, the three regions (the Middle East, Northeast Asia, and Southeast Asia) increased their production of toluene over the same time frame. In addition, demand in developing regions such as China and Thailand saw continued growth during this period [1]. Vietnam, Malaysia, Indonesia, Thailand, and the Philippines are fast growing South East Asian furniture suppliers. The development of these five countries furniture production has been export driven. Thailand's labor force was estimated at 36.9 million in 2012. About 49% were employed in agriculture, 37% in the service sector, and 14% in industry [2]. Because exporting Thai furniture and related products is big business and an area with growth potential, Thailand's Ministry of Commerce and the Department of Export Promotion are working to establish Thailand as an Asian hub for furniture product exports. Main Thai furniture and related export items include wood furniture (50.17%), others (22.82%), beds, mattresses, pillows (5.31%), and metal furniture (5.31%). Major steel furniture export market for Thailand in 2008 were the USA, Japan, the United Kingdom, Australia, and Germany, representing 62% of the total value of furniture export [3]. Currently in Thailand, there are over 46 steel furniture factories with approximately 5,500 employees [2]. Employees' work included cutting, bending, and welding sheet metal to form end of products. Some employees reported breathing problems, excessive tiredness, and dust in their noses at the end of their work shifts. After welding, employees in the grinding area used hand-held grinders to smooth the welds and remove sharp edges. Completed parts traveled on an overhead conveyor through a degreasing booth, where they were rinsed with phosphoric acid. As the parts exited the booth, an employee used a rag dipped in toluene for touch-up cleaning [4]. Repeatedly breathing toluene over long periods of time at work, or through deliberately “sniffing” or “huffing” glue or paint, can cause death, permanent brain damage, or depression [5].
Metal furniture is a type of furniture that uses metal parts in its construction. There are various types of metal that can be used, such as iron, aluminum, and stainless steel. Steel furniture is preferred over other kinds of furniture due to its durability, fold ability (in many cases), and easy transportability. The manufacturing process of steel furniture involves 10 main activities: cutting of sheets, tubes, and flats to desired size; and folding, bending, drilling, punching, riveting, and assembling as per the drawings. Finally, the items are spray painted [6]. Spray painters have to step into the booth to touch up areas on parts that were missed by the mounted spray paint guns. This could increase their exposure to triglycidyl isocyanurate and dust [7]. Solvents are added to coatings to disperse the other constituents of the formulation and to reduce viscosity, thereby enabling application of the coating. A wide variety of solvents are used in paints, including aliphatic hydrocarbons, aromatic hydrocarbons (toluene, xylene, and the trimethyl benzenes), ketones [methyl ethyl ketone (MEK) and methyl isobutyl ketone], alcohols, esters, and glycol ethers [8]. Because organic solvents are ingredients of many products such as paints and cleaning agents, they are also found in manufacturing workplaces and work settings. Thus, workers may have repeated exposure to very high organic solvent levels many times during the course of their working lives [9]. Workers exposed to solvents are at risk of developing a chronic toxic encephalopathy, although effects (promoting or etiological) on other central and peripheral nervous system diseases may be possible [10]. Companies also add toluene to aerosol spray paints, wall paints, lacquers, paint strippers, adhesives, printing ink, spot removers, cosmetics, perfumes, and antifreeze [11–13].
Toluene can be absorbed into the blood via the lungs, the gastrointestinal tract, through the skin and mucosa. In addition, toluene accumulated in adipose tissue when absorbed by skin or ingested due to its hydrophilic properties [8]. More than 80% of absorbed toluene is metabolized by mixed-function oxidase enzyme systems into benzoic and hippuric acid prior to excretion into the urine. Some absorbed toluene (0.4–1.1%) is hydroxylated and excreted as a mixture of ortho-, para-, and meta-cresol [14–16]. More than 75% is eliminated within 12 hours after exposure [17]. Among the metabolites, hippuric acid is a traditional biomarker in the biological monitoring of occupational exposure to toluene [18,19].
Hippuric acid is still the most used indicator in the monitoring of toluene in exposed workers, as it shows a good correlation with exposure levels, despite its lack of specificity to toluene, or occupational exposure [20]. Toluene is metabolized to hippuric acid via benzoic acid in the human body [21,22]. Therefore, the most important sources of background hippuric acid are environmental toluene contamination and dietary food, such as fruit (plum and peach), green coffee beans and other benzoic acid liberators [15,23]. Acute and chronic exposure is known to cause multiple effects in the body. It affects the central nervous, cardiovascular, renal, and gastrointestinal systems. It also produces severe acid-base and electrolyte disturbances. It has been noted that chronic toluene abusers present with generalized muscle weakness and develop acute respiratory failure [24–27].
Many occupations have a high risk of toluene exposure. Burgaz et al [28] reported that people employed in the shoe manufacture and repair industry, who were exposed to organic solvents, mainly toluene, were at an increased risk of cancer, with the strongest evidence being for nasal cancer and leukemia. Inhalation of high levels of toluene vapor for a short period may cause drowsiness, headache, nausea, visual changes, muscle spasm, dizziness, and loss of coordination [16].
The aims of this study were to investigate the occupational exposure to airborne toluene in paint workers in the manufacture of steel furniture, to determine the hippuric acid levels in their urine samples, to describe the workers' hygiene behaviors, health symptoms, and to make clear whether relationships between airborne toluene and urine hippuric acid concentrations would be useful for assessing workers toluene exposure.
2. Materials and methods
In this cross-sectional study, urinary samples were collected from workers in three paint-management factories in Thailand, during September–November 2012. This research was approved by the Ethics Committee of the Institute of Research and Development, Thaksin University, Phattalung, Thailand.
2.1. Study population and samples
The study population comprised an exposed group and a control group. The exposed group was made up of 87 paint workers and workers from three paint-management factories in Thailand. The study enrolled workers at painting sites with records of high exposures. The inclusion criteria for the exposed group were: painting workers and workers in occupational contact with the aerosol spray paints during work, who had worked with that occupational contact for at least 1 year. They were aged 20–60 years. They agreed to participate in the study, and provided informed written consent. Of the 87 exposed workers recruited into the study, 53 were male and 34 female. The nonexposed group (87 volunteers) was selected from the general population living in the same area as the factories, and comprised people who did not have occupational contact with the aerosol spray paints during work. They were matched for sex with the exposed group.
2.2. Sample collection
The 174 participants (87 exposed; 87 nonexposed) were interviewed using a questionnaire. General information on the paint workers was collected by face-to-face interview using a survey form, and by walk-through on-site survey. Urine samples of both the 87 exposed and 87 nonexposed workers were collected before shift on the first working day, and at the end of shift on the second working day.
2.3. Questionnaire
In the questionnaire, information on the following variables was collected over 7 days: general information; personal hygiene; and the development of acute symptoms. Acute symptoms were short-term toluene postexposure symptoms that appeared to result from working with aerosol spray paint. Direct observation was also used to confirm the interview results.
2.4. Airborne toluene collection
For each examined worker, exposure was measured in their breathing zone with personal passive dosimeters (TK-200; Zambelli, Bologna, Italy). The whole work-shift (8 hours) of Thursday was evaluated, each sampling period lasting 8 hours.
2.5. Urine collection
Spot urine samples (50 mL) were collected in polyethylene bottles, and creatinine was tested prior to acidification and frozen at −20°C until analysis (within 4 days after sampling).
2.6. Laboratory analysis
Toluene in the breathing zone and hippuric acid in urine were both determined by gas and liquid chromatography, respectively.
2.6.1. Determination of toluene concentration in the breathing zone air
The time–weight average (TWA) exposure of each worker to toluene was measured by a diffusive sampler with carbon cloth (Toyobo Co., Osaka, Japan). After exposure, the carbon cloth toluene extract was measured by flame ionization detection gas–liquid chromatography technique (Model GC-148; Shimadzu, Tokyo, Japan) using a DB-1 capillary column (30 m × 0.53 mm inner diameter; J&W Scientific, Folsom, CA, USA).
2.6.2. Determination of hippuric acid concentration in urine
Solutions of hippuric acid (Sigma–Aldrich, St Louis, MO, USA) were prepared at concentrations of 10 g/L and 5 g/L. An internal standard solution, 0.4 g/L heptadecanoic acid (Sigma–Aldrich), was prepared in methanol. Hippuric acid extraction from urine was performed according to method of Kira et al [29], and identified using the Alvarez-Leite et al [30] gas–liquid chromatography technique using a DB-1 capillary column (30 m × 0.53 mm inner diameter; J&W Scientific) and flame ionization detector with an oven temperature of 200°C, injector and detector temperature of 250°C and a helium flow rate of 10 mL/minute. Calibration curves were prepared adding scaled quantities of hippuric acid in urine to obtain concentrations of 0.1 g/L, 0.2 g/L, 0.5 g/L, 0.8 g/L, 1.0 g/L, 1.2 g/L, and 1.5 g/L (r = 0.9934). Three calibrators were analyzed daily (0.1 g/L, 0.5 g/L, and 1.0 g/L) along with the assay of samples to be evaluated. The lower quantification limit for hippuric acid in urine was 0.05 g/L. The assay precision was determined by analyzing samples containing hippuric acid at 0.2 g/L, 1.0 g/L, and 1.5 g/L. The intra-assay variation coefficient was 8.2% (10 samples for each concentration) and interassay variation was 10.2% (for samples analyzed daily during 10 days). Urinary creatinine concentrations were determined using the kinetic Jaffe colorimetric method using picric acid [31].
2.7. Statistical analysis
Descriptive statistics were used to display the hippuric acid concentration results in urine. The Mann–Whitney U test was used to compare the medians of continuous variables of the exposed and control groups. Spearman's rank correlation test was used to test the association of independent factors with the hippuric acid concentrations in biological samples. The medians of data were compared using the Mann–Whitney U test for two groups or the Kruskal–Wallis test for more than two groups.
3. Results
3.1. General characteristics of the participants
There were 174 participants in the present study. Most were aged 20–34 years, at 69.0% (exposed) and 66.7% (nonexposed), respectively. Most of the exposed participants were married, whereas most of the controls were single. All were Buddhists. Most of the exposed participants had secondary level education, whereas the controls had diploma or equivalent level. More exposed participants smoked cigarettes and drank alcoholic beverages than did the controls (Table 1).
Table 1.
General characteristics of the study participants
| Parameter | Nonexposed (n = 87) | Exposed (n = 87) |
|---|---|---|
| Gender | ||
| Male | 53 (60.9) | 53 (60.9) |
| Female | 34 (39.1) | 34 (39.1) |
| Age (y) | ||
| 20–34 | 60 (69.0) | 58 (66.7) |
| 35–44 | 25 (28.7) | 24 (27.6) |
| 45–54 | 2 (2.3) | 5 (5.7) |
| Education level | ||
| Secondary school or equivalent | 18 (20.7) | 69 (79.3) |
| Diploma or equivalent | 53 (60.9) | 3 (3.4) |
| Bachelor degree or higher | 16 (18.4) | 15 (17.3) |
| Marital status | ||
| Single | 16 (18.4) | 41 (47.10) |
| Married | 48 (55.2) | 31 (35.6) |
| Widowed | 9 (10.3) | 7 (8.1) |
| Separated/divorced | 14 (16.1) | 8 (9.2) |
| Smoke cigarettes | ||
| Yes | 21 (24.1) | 52 (59.8) |
| No | 66 (75.9) | 35 (40.2) |
| Consume alcohol | ||
| Yes | 15 (17.3) | 50 (57.5) |
| No | 72 (82.7) | 37 (42.5) |
Values are presented as n (%).
3.2. Airborne toluene levels and hippuric acid in urine levels of the participants
The airborne toluene levels that the paint workers were exposed to are shown in Table 2.The median hippuric acid in urine level of the exposed and control subjects was significantly different (p < 0.001). The median hippuric acid level in urine of the exposed workes was 800 mg/g creatinine (range, 90–2,547 mg/g creatinine). Thirteen of 87 exposed participants (14.9%) had levels that exceeded the accepted safe standard (1,600 mg/g creatinine, biological exposure index) recommended by the American Conference of Governmental Industrial Hygiene (ACGIH) [32].
Table 2.
Airborne toluene levels and hippuric acid in urine levels of the participants
| Parameter | Median | Interquartile range | Range | p |
|---|---|---|---|---|
| Air borne toluene levels (ppm; n = 87) | 55 | 87 | 12–198 | |
| Hippuric acid in urine levels (mg/g creatinine) | ||||
| Nonexposed controls (n = 87) | 200 | 210 | 50–580 | < 0.001 |
| Exposed workers (n = 87) | 800 | 669 | 90–2547 | |
3.3. Correlation between hippuric acid and airborne toluene levels
Fig. 1 shows the correlation between the airborne toluene levels (personal sampling) and the hippuric acid levels of the study group. The relationship between airborne toluene and hippuric acid levels was significant (r = 0.548, p < 0.01).
Fig. 1.
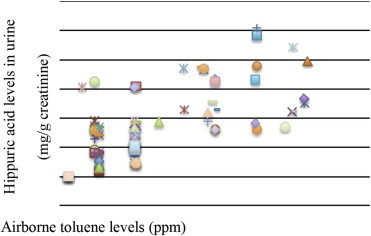
The correlation plot of airborne toluene levels (personal sampling) versus paint workers' hippuric acid levels.
3.4. General characteristics and hippuric acid levels
General characteristics of gender, age, education level, education levels, marital status, cigarette smoking, or alcohol consumption, and their relation to hippuric acid levels were not significantly statistically different between the two groups.
3.5. Occupational lifestyles, personal protective equipment and personal hygiene, acute symptoms, and hippuric acid levels in urine of the exposed workers
For those working in steel furniture manufacture, those who worked and used the same places during their work breaks had significantly higher urinary hippuric acid levels than those who did not. All workers worked > 9 hours/day in the paint process/area. Most workers (75.9%) used a filter mask to protect themselves during work. Seventy-seven percent (77%) of workers used gloves, 12.7% did not use protective clothing (aprons) when spaying paint, and 16.1% ate snacks or drank water while working. Most of the workers (85.1%) washed their hands prior to lunch and 82.8% did not change their clothes prior to going home. It was found that median hippuric acid levels and the use of personal protective equipment (PPE; filter mask and aprons), eating snacks or drinking water during work, and changing clothes, after work had significantly significant correlations (p < 0.05; Table 3). Workers who had worked > 5 years had significantly higher hippuric acid levels than those who had worked < 5 years (p < 0.026). Workers who used a filter mask and/or wore aprons, had significantly lower hippuric acid levels than those who did not (p < 0.001 for both). Workers who ate snacks while working had significantly higher hippuric acid levels than those who did not (p < 0.033). Workers who changed clothes after work had significantly lower hippuric acid levels than those who did not (p < 0.045). Most nonexposed participants reported no acute or chronic symptoms. Among the exposed workers, 44.8% reported headache, 48.3% reported dizziness, and 56.3% reported nausea, respectively. The hippuric acid levels of participants who reported and who did not report acute symptoms were compared. The exposed workers who reported symptoms of headache, dizziness, and nausea had significantly higher hippuric acid levels than those who did not have symptoms (p < 0.001 for all). The exposed workers who reported muscle spasm had significantly higher hippuric acid levels than those did not report this symptom (p ≤ 0.001; Table 4).
Table 3.
Comparison between hippuric acid levels and occupational lifestyles, personal protective equipment and personal hygiene of the exposed workers
| Parameter | Number of exposed workers (%) | Median of hippuric acid (mg/g creatinine) | Interquartile range | Range | p |
|---|---|---|---|---|---|
| Duration of work (y) | |||||
| ≤ 5 | 7 (8.0) | 560 | 610 | 1150–160 | 0.026* |
| > 5 | 80 (92.0) | 800 | 688 | 2547–90 | |
| Personal protective equipment | |||||
| Filter mask | |||||
| No | 21 (24.1) | 1660 | 455 | 1111–2547 | <0.001* |
| Yes | 66 (75.9) | 655 | 440 | 90–1150 | |
| Gloves | |||||
| No | 20 (23.0) | 800 | 1120 | 90–1895 | 0.242 |
| Yes | 67 (77.0) | 800 | 690 | 160–2547 | |
| Aprons | |||||
| No | 11 (12.7) | 1260 | 1045 | 150–2547 | <0.001* |
| Yes | 76 (87.3) | 740 | 512 | 90–1660 | |
| Personal hygiene | |||||
| Ate snacks or drank water at work | |||||
| No | 73 (83.9) | 730 | 590 | 90–2547 | 0.033* |
| Yes | 14 (16.1) | 940 | 584 | 220–1830 | |
| Washed hands before lunch | |||||
| No | 13 (14.9) | 710 | 735 | 150–2420 | 0.211 |
| Yes | 74 (85.1) | 800 | 720 | 90–2547 | |
| Changed cloths after work | |||||
| No | 72 (82.8) | 900 | 782 | 150–2547 | 0.045* |
| Yes | 15 (17.2) | 440 | 400 | 90–1660 | |
Table 4.
Comparison between hippuric acid levels and reported acute neurologic symptoms
| Parameter | Number of exposed workers | Median of hippuric acid (mg/g creatinine) | Interquartile range | Range | p |
|---|---|---|---|---|---|
| Headache | |||||
| No | 48 | 545 | 478 | 90–1280 | < 0.001* |
| Yes | 39 | 1145 | 824 | 220–2547 | |
| Dizziness | |||||
| No | 45 | 460 | 550 | 90–1875 | < 0.001* |
| Yes | 42 | 945 | 850 | 220–2547 | |
| Nausea | |||||
| No | 38 | 450 | 532 | 90–2420 | < 0.001* |
| Yes | 49 | 860 | 640 | 420–2547 | |
| Anorexia | |||||
| No | 55 | 790 | 520 | 90–1280 | 0.117 |
| Yes | 32 | 800 | 900 | 220–2547 | |
| Insomnia | |||||
| No | 39 | 790 | 530 | 90–1895 | 0.063 |
| Yes | 48 | 840 | 1000 | 160–2547 | |
| Loss of coordination | |||||
| No | 83 | 800 | 688 | 90–1895 | 0.974 |
| Yes | 4 | 840 | 860 | 160–2547 | |
| Muscle spasm | |||||
| No | 50 | 545 | 472 | 90–1895 | < 0.001* |
| Yes | 37 | 1145 | 812 | 220–2547 | |
Hippuric acid levels in urine (mg/g creatinine) Airborne toluene levels (ppm).
4. Discussion
4.1. Airborne toluene levels and hippuric acid in urine
Air sampling is commonly used to evaluate occupational exposure to toluene. The major metabolic pathway is oxidation of the side chain to benzoic acid, followed by conjugation with glycine to hippuric acid (about 80%). Hippuric acid excretion has previously been used for biological monitoring [33]. However, hippuric acid is also excreted in varying amounts in people not exposed to toluene, as a metabolite of benzoic acid, irrespective of origin [34]. Maria et al [15] reported that approximately 70% of the food items tested from the Philippines were positive for sodium benzoate. The values of the compound in the Philippines samples range of 20 μg/mL to > 2,000 μg/mL; the Japanese products showed a range of 50–200 μg/mL. Hippuric acid levels expressed as geometric mean were 0.11 g/g creatinine (0.41) for the Filipino subjects and 0.09 g/g creatinine (0.39) for the Japanese.
The median airborne toluene levels were 55 ppm (range, 12–198 ppm). The TWA of all airborne toluene levels were < 200 ppm [35], the limiting value level for concerned by Occupational Safety and Health Administration. Therefore, the traditional biomarker of hippuric acid is valid when TWA toluene exposure is rather intense [36], this marker only poorly correlated with the exposure when toluene exposure is low, e.g., < 10 ppm [37]. Thus, the value of o-cresol also appears to be limited under low toluene exposure.
Among workers in the present study, 14.9% of paint workers had hippuric acid levels of > 1.6 g/g creatinine [32], the ACGIH-recommended biological exposure index for hippuric acid in urine. There are some reports concerning the stability of toluene in toluene/blood samples in blood collecting vessels. However, studies using blood samples from toluene-exposed paint workers, in the context of medical examinations, are lacking. In this study we measured hippuric acid levels in urine, because hippuric acid is a byproduct of toluene that may be used as an indirect measure of toluene levels [19]. The results of the present study showed that the hippuric acid levels in these paint workers were higher than the matched controls. Among the paint workers in the present study, the median hippuric acid level was 800 mg/g creatinine (range, 90–2547 mg/g creatinine). This study found a correlation between airborne toluene and hippuric acid levels (r = 0.548, p < 0.010). This result agreed with Truchon et al [38], who reported the 8-hour toluene exposure (TWA) ranged from 0 ppm to 111 ppm. Toluene exposure was well correlated to postshift urinary o-cresol (r = 0.89) and hippuric acid (r = 0.67) levels. At low exposure levels (< 50 ppm), however, o-cresol shows a stronger correlation (r = 0.71) than hippuric acid (r = 0.24). Urinary hippuric acid may not be effective when exposure is below a certain level. This result is supported by the observation of Nise G [39] that hippuric acid excretion is unsuitable for biological monitoring of toluene exposure when the exposure level is < 200 mg/m3. This complies with the requirement of ACGIH [32] that replaced hippuric acid with o-cresol or urinary toluene for the biomarker of toluene exposure.
4.2. Factors associated with urinary hippuric acid levels
A recent study found that many factors influence increased hippuric acid levels. Cigarette smoking enhanced elimination of toluene and hippuric acid from the body. However, cigarette smoking and its relationship to hippuric acid levels showed no statistically significant difference between the two groups. The present study differs from Wang et al [40], who reported the blood toluene in the occupationally nonexposed general population. In that study, the smokers had significantly higher blood toluene levels than nonsmokers, with the level affected more by the length of time since the last cigarette was smoked, than by the extent of smoking. With regard to working duration, it was found that median hippuric acid levels differed significantly; workers who had worked ≥ 5 years had significantly higher hippuric acid levels compared to those who had worked < 5 years.
The types of PPE in use in these factories were inappropriate for this type of work. Toluene can accumulate on the surfaces of the PPE used by the paint workers. In addition, toluene may penetrate a cotton mask and enter a worker's airway. Paint workers using these inappropriate protective devices may also mistakenly believe that they are protected. With regard to PPE use, it was found that workers who used filter masks and aprons had significantly lower urinary mercury levels than those who did not. Paint workers who had poor protective practices had a urinary hippuric acid level up to 2547 mg/g creatinine (range, 90–2547 mg/g creatinine). The authors noted that a painting worker regularly entered the spray booth and used a squeegee to remove paint overspray that had settled onto the floors and walls of the booth. This painting worker was exposed to up to 2547 ppm of toluene for 8 hours/day, 6 days/week for 26 years. The worker normally did not use a filter mask and aprons and had poor personal hygienic practice and was therefore the highest exposed worker of the group. Also, 14.9% of paint workers had hippuric acid levels of > 1.6 g/g creatinine. They did not use PPE at work and practiced poor hygiene, such as not washing their hands prior to and after work. Thus, self-protection is important, because the risk of exposure to hazardous materials can be reduced by implementation of appropriate behaviors. This result was supported by Rogers [41].
Workers who ate snacks or drank water while working had significantly higher hippuric acid levels than those who did not. Paint workers who changed their clothes after work, had significantly lower hippuric acid levels than those who did not change their clothes. Paint workers often did not change clothes prior to going home. These poor protective practices meant that paint workers were likely to carry toluene contamination elsewhere, potentially exposing their homes and families. Paraoccupational asthma has also been described, e.g., cases of asthma caused by exposure to toluene di-isocyanate in people not directly working with this chemical but working in the vicinity of factories using toluene di-isocyanate [42]. Paraoccupational exposure of children via parent(s) to asbestos, pesticides, and organic solvents with health sequelae have been described [21,43]. There is also evidence that occupational allergens can be transported home, presumably on contaminated clothing and skin, with subsequent atopic sensitization of other household residents [44–46]. Disorders of neurological and systemic function can occur, as reported by several studies [12]. Acute narcotic effects on central nervous system, including headache [47,48], dizziness [48,49], nausea [50], and muscle spasm, were significantly higher among those with higher hippuric acid levels. The main pathway for raising hippuric acid levels among paint workers is probably the inhalation route. This is supported by the Agency for Toxic Substances and Disease Registry [17].
Toluene vapor is only mildly irritating to mucous membranes; however, toluene absorbed through the skin may also contribute to total body burden. In addition, Burgaz et al [28] reported that the cytogenetic damage related to occupational exposure to airborne chemicals during shoemaking and the processes in pathology and anatomy laboratories, the micronuclei (MN) count per 3000 cells was measured in buccal smears from shoe workers (group I, n = 22) exposed to mainly n-hexane, toluene and MEK and from anatomy and pathology staff (group II, n = 28) exposed to formaldehyde. Eighteen male university staff were used as controls. The mean ± standard deviation micronuclei frequencies in buccal mucosa cells from workers in Group I, Group II, and controls were 0.62 ± 0.45%, 0.71 ± 0.56%, and 0.33 ± 0.30%, respectively (p < 0.05 for both compared with controls for Group I and Group II). Thus, occupational exposure to organic solvents, mainly n-hexane, toluene, MEK, and formaldehyde, may cause cytogenetic damage in buccal cells. However, epidemiologic studies of workers exposed to toluene (or toluene together with other solvents) have not demonstrated increased risks for cancer, and animal studies have not demonstrated an increased incidence of tumors. The International Agency for Research on Cancer determined that toluene was not classifiable with regard to human carcinogenicity [51]. Toluene contamination of hands, clothes, and work surfaces can occur. Poor hygiene behaviors may therefore be associated with elevated hippuric acid levels. This is also supported by Hawkins and Evans [52], who reported the subjective estimation of toluene exposures. Results suggest that experienced hygienists may be competent at providing subjective estimates of average exposures for retrospective epidemiological studies and prospective hazard evaluation. Thus, elevated hippuric acid levels might result from inappropriate behaviors, such as hand–mouth contact with infrequent hand-washing prior to eating and drinking. In addition, headache, dizziness, nausea, and muscle spasm at least once per week were significantly higher among those with elevated urinary hippuric acid levels. Good personal hygienic practice may be supplemented to control the hippuric acid levels in paint workers.
In conclusion, paint workers are exposed to toluene. Urinary hippuric acid, a marker of toluene exposure, has a short half-life and a good relationship with high levels of toluene exposure. Thus, workers with high levels of hippuric acid are at increased risk of developing neurological symptoms. However, although urine sampling is more convenient and less invasive than blood sampling to screen for toluene exposure, there may be situations in which blood sampling for toluene is more useful. The present study had some limitations. The sample size was relatively small, and it was difficult to obtain cooperation. Therefore, further research in this field needs to be done, e.g., with a larger sample size. Given that environmental toluene is toxic to humans, minimizing potential exposure is advisable. This study showed that improving painting workers' hygiene habits can reduce their hippuric acid levels. Thus, we suggest that the tasks and behaviors at work that expose workers to lead should be assessed, and correct work practices and safety behaviors of painting workers including good personal hygienic practice be encouraged.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors would like to thank all the people who participated in, and cooperated with, this study, and for allowing us to collect urine and airborne toluene samples. Sincere appreciation is also extended to all staff of the Central Equipment Unit, Faculty of Tropical Medicine, Mahidol University.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/ licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.IHS Chemical. Toluene [Internet]. 2013 [cited 2014 Feb 9]. Available from: http://www.ihs.com/products/chemical/planning/ceh/toluene.aspx.
- 2.Office of the National Economic and Social Development Board. The Economic Performance in Q1 and Outlook for 2013 [Internet]. 2013 [cited 2014 Mar 28]. Available from: http://www.nesdb.go.th/.
- 3.Department of International Trade Promotion. Thailand's Furniture Industry [Internet]. 2014 [cited 2014 Mar 28]. Available from: http://www.thaitradeusa.com/home/?page_id=2729.
- 4.Brueck SE, Ramsey J, Rodriguez M, Adebayo A. NIOSH puts health hazard to bed and metal furniture [Internet]. 2013 [cited 2014 Mar 28]. Available from: http://ehstoday.com/health/niosh-puts-health-hazards-bed-metal-furniture-manufacturer.
- 5.U.S. National Library of Medicine. Toluene [Internet]. 2013 [cited 2014 Mar 28]. Available from: http://toxtown.nlm.nih.gov/text_version/chemicals.php?id=30.
- 6.Steel furniture [Internet]. 2011 [cited 2014 Feb 9]. Available from: http://smallb.in/sites/default/files/knowledge_base/steel_furniture.pdf.
- 7.Allmaras S. Worker exposure to 1,3,5-triglycidyl isocyanurate (TGIC) in powder paint coating operations. Appl Occup Environ Hyg. 2003;1:151–153. doi: 10.1080/10473220301354. [DOI] [PubMed] [Google Scholar]
- 8.U.S. National Library of Medicine. Tox Tone [Internet]. 2014 [cited 2014 Feb 11]. Available from: http://toxtown.nlm.nih.gov/text_version/chemicals.php?id=30.
- 9.Department of Health and Human Services, Participating Institutes and Centers (ICs). Neurological indices of long term solvent exposure in workers [Internet]. 2003 [cited 2014 Feb 11]. Available from: http://grants.nih.gov/grants/guide/rfa-files/RFA-OH-04-001.html.
- 10.Viaene M.K. Overview of the neurotoxic effects in solvent-exposed workers. Arch Public Health. 2002;60:217–232. [Google Scholar]
- 11.Sourindrhin I. Solvent misuse. Br Med J. 1985;290:92–95. doi: 10.1136/bmj.290.6462.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameno K., Fuke C., Ameno S., Kiriu T., Sogo K., Ijiri I. A fatal case of oral ingestion of toluene. Forensic Sci Int. 1989;41:255–260. doi: 10.1016/0379-0738(89)90218-1. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda N., Takahashi H., Umetsu K., Suzuki T. The course of respiration and circulation in ‘toluene sniffing’. Forensic Sci Int. 1990;44:151–158. doi: 10.1016/0379-0738(90)90245-t. [DOI] [PubMed] [Google Scholar]
- 14.Amorim L.C.A., Alvarez-Leite E.M. Determination of o-cresol by gas chromatography and comparison with hippuric acid levels in urine samples of individuals exposed to toluene. J Toxicol Environ Health. 1997;30:101–107. [PubMed] [Google Scholar]
- 15.Maria B.G., Villanueva M.B., Jonai H., Kainno S., Takeuchi Y. Dietary sources and background levels of hippuric acid in urine: comparison of Philippine and Japanese levels. Ind Health. 1994;32:239–246. doi: 10.2486/indhealth.32.239. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki K., Tanaka E., Misawa S. Urinary ortho-cresol concentrations as an indicator of toluene inhalation in glue sniffers. J Forensic Sci Soc. 1992;32:215–223. doi: 10.1016/s0015-7368(92)73074-6. [DOI] [PubMed] [Google Scholar]
- 17.Agency for Toxic Substances and Disease Registry . ATSDR; Atlanta: 2000. Toxicological profile for toluene (Update) [PubMed] [Google Scholar]
- 18.Apostoli P., Brugnone F., Perbellini L., Cocheo V., Bellomo M.L., Silvestri R. Biomonitoring of occupational toluene exposure. Int Arch Occup Environ Health. 1982;50:153–168. doi: 10.1007/BF00378077. [DOI] [PubMed] [Google Scholar]
- 19.De Rosa E., Brugnone F., Bartolucci G.B., Perbellini L., Bellomo M.L., Gori G.P., Sigon M., Chiesura Corona P. The validity of urinary metabolites as indicators of low exposures to toluene. Int Arch Occup Environ Health. 1985;56:135–145. doi: 10.1007/BF00379385. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Leite E.M., Duarte A., Barroca M.M., Silveira N.S. Possible effects of drinking and smoking habits on hippuric acid levels in urine of adults with no occupational toluene exposure. J Occup Health. 1999;41:112–114. [Google Scholar]
- 21.McDiarmid M.A., Weaver V. Fouling one's own nest revisited. Am J Ind Med. 1993;24:1–9. doi: 10.1002/ajim.4700240102. [DOI] [PubMed] [Google Scholar]
- 22.Anderson C.E., Loomis G.A. Recognition and prevention of inhalant abuse. Am Fam Physician. 2003;68:869–874. [PubMed] [Google Scholar]
- 23.Kawamoto T., Koga M., Oyama T., Kodama Y. Habitual and genetic factors that affect urinary background levels of biomarkers for organic solvent exposure. Arch Environ Contam Toxicol. 1996;30:114–120. doi: 10.1007/s002449900015. [DOI] [PubMed] [Google Scholar]
- 24.Tang H.L., Chu K.H., Cheuk A., Kodama Y. Renal tubular acidosis and severe hypophosphataemia due to toluene inhalation. Hong Kong Med J. 2005;11:50–53. [PubMed] [Google Scholar]
- 25.Kao K.C., Tsai Y.H., Lin M.C., Huang C.C., Tsao C.Y., Chen Y.C. Hypokalemic muscular paralysis causing acute respiratory failure due to rhabdomyolysis with renal tubular acidosis in a chronic glue sniffer. J Toxicol Clin Toxicol. 2000;38:679–681. doi: 10.1081/clt-100102021. [DOI] [PubMed] [Google Scholar]
- 26.Lavoie F.W., Dolan M.C., Danzl D.F., Barber R.L. Recurrent resuscitation and ‘no code’ orders in a 27-year-old spray paint abuser. Ann Emerg Med. 1987;16:1266–1273. doi: 10.1016/s0196-0644(87)80237-8. [DOI] [PubMed] [Google Scholar]
- 27.Gehle K, Pharagood-Wade F, Johnson D, Rosales-Guevara L. Toluene toxicity. Case studies in environmental medicine [Internet]. Agency for Toxic Substances and Disease Registry (ATSDR). 2014 [cited 2014 Mar 28]. Available from: http://www.atsdr.cdc.gov/csem/toluene/docs/toluene.pdf.
- 28.Burgaz S., Erdem O., Cakmak G., Erdem N., Karakaya A., Karakaya A.E. Cytogenetic analysis of buccal cells from shoe-workers and pathology and anatomy laboratory workers exposed to n-hexane, toluene, methyl ethyl ketone and formaldehyde. Biomarkers. 2002;7:151–161. doi: 10.1080/13547500110113242. [DOI] [PubMed] [Google Scholar]
- 29.Kira S. Measurement by gas-chromatography of urinary hippuric acid and methyl hippuric acid as indices of toluene and xylene exposures. Brit J Ind Med. 1977;34:305–309. doi: 10.1136/oem.34.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Leite E.M., França L.S., Ribeiro L.B. Determination of urinary hippuric acid by gas-chromatography after derivatization with trimethylphenylammonium hydroxide. Toxirama. 1994;6:3–6. [Google Scholar]
- 31.Smith S.T. Nonprotein. In: Bishop M.L., Duben-Von Laufen J.L., Fody E.P., editors. Clinical chemistry: principles, procedure, correlations. Lippincott; Philadelphia: 1985. pp. 415–416. [Google Scholar]
- 32.American Conference of Governmental Industrial Hygienists . 2014. Threshold limit values and biological exposure indices for 2014. Cincinnati. [Google Scholar]
- 33.Droz P.O., Berode M., Biollat M.A., Lob M. Biological monitoring and health surveillance of rotogravure printing workers exposed to toluene. In: Ho M.H., Dillon H.K., editors. Biological monitoring of exposure to chemicals. Wiley; New York: 1987. pp. 111–131. [Google Scholar]
- 34.Kawai T., Yasugi T., Mizunuma K., Horiguchi S., Ikeda M. Comparative evaluation of blood and urine analysis as a tool for biological monitoring of n-hexane and toluene. Int Arch Occup Environ Health. 1993;65(1 Suppl):S123–S126. doi: 10.1007/BF00381322. [DOI] [PubMed] [Google Scholar]
- 35.Occupational Safety & Health Administration . NW; Washington, DC: 1989. OSHA PEL – construction industry. [Google Scholar]
- 36.Ukai H., Kawai T., Inoue O., Maejima Y., Fukui Y., Ohashi F., Okamoto S., Takada S., Sakurai H., Ikeda M. Comparative evaluation of biomarkers of occupational exposure to toluene. Int Arch Occup Environ Health. 2007;81:81–93. doi: 10.1007/s00420-007-0193-0. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T., Ukai H., Inoue O., Maejima Y., Fukui Y., Ohashi F., Okamoto S., Takada S., Sakurai H., Ikeda M. Evaluation of biomarkers of occupational exposure to toluene at low levels. Int Arch Occup Environ Health. 2008;81:253–262. doi: 10.1007/s00420-007-0203-2. [DOI] [PubMed] [Google Scholar]
- 38.Truchon G., Tardif R., Brodeur J. o-Cresol: a good indicator of exposure to low levels of toluene. Appl Occup Environ Hyg. 1999;14:677–681. doi: 10.1080/104732299302297. [DOI] [PubMed] [Google Scholar]
- 39.Nise G. Urinary excretion of o-cresol and hippuric acid after toluene exposure in otogravure printing. Int Arch Occup Environ Health. 1992;63:377–381. doi: 10.1007/BF00386931. [DOI] [PubMed] [Google Scholar]
- 40.Wang G., Maranelli G., Perbellini L., Guglielmi G., Brugnone F. Reference values for blood toluene in the occupationally nonexposed general population. Int Arch Occup Environ Health. 1993;65:201–203. doi: 10.1007/BF00381156. [DOI] [PubMed] [Google Scholar]
- 41.Rogers R.W. Cognitive and physiological processes in fear appeals and attitude change: revised theory of protection motivation. In: Cacioppo J., Petty R., editors. Social psychophysiology. Guilford Press; New York (NY): 1983. pp. 153–176. [Google Scholar]
- 42.De Zotti R., Muran A., Zambon F. Two cases of paraoccupational asthma due to toluene diisocyanate (TDI) Occup Environ Med. 2000;57:837–839. doi: 10.1136/oem.57.12.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnusson L.L., Wennborg H., Bonde J.P., Olsen J. Wheezing, asthma, hay fever, and atopic eczema in relation to maternal occupations in pregnancy. Occup Environ Med. 2006;63:640–646. doi: 10.1136/oem.2005.024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alonso E., Ausin A., Elices A., Moreno-Escobosa M., Ibáñez M., Laso M. Baker's asthma in a child. Allergol Immunopathol (Madr) 2001;29:141–143. doi: 10.1016/s0301-0546(01)79048-9. [DOI] [PubMed] [Google Scholar]
- 45.Venables K., Newman-Taylor A.J. Asthma related to occupation of spouse. Practitioner. 1989;233:809–810. [PubMed] [Google Scholar]
- 46.Tagiyeva N., Devereux G., Semple S., Sherriff A., Henderson J., Elias P., Ayres J.G. Parental occupation is a risk factor for childhood wheeze and asthma. Eur Respir J. 2010;35:987–993. doi: 10.1183/09031936.00050009. [DOI] [PubMed] [Google Scholar]
- 47.Iregren A., Akerstedt T., Anshelm Olson B., Gamberale F. Experimental exposure to toluene in combination with ethanol intake: psychophysiological functions. Scand J Work Environ Health. 1986;12:128–136. doi: 10.5271/sjweh.2167. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y.L., Pai M.C., Chen J.H., Guo Y.L. Central neurological abnormalities and multiple chemical sensitivity caused by chronic toluene exposure. Occup Med. 2003;53:479–482. doi: 10.1093/occmed/kqg095. [DOI] [PubMed] [Google Scholar]
- 49.Welch L., Kirshner H., Heath A., Gilliland R., Broyles S. Chronic neuropsychological and neurological impairment following acute exposure to a solvent mixture of toluene and methyl ethyl ketone (MEK) Clin Toxicol. 1991;29:435–445. doi: 10.3109/15563659109025739. [DOI] [PubMed] [Google Scholar]
- 50.Rogers S.A. Diagnosing the tight building syndrome or diagnosing chemical hypersensitivity. Environ Int. 1989;15:75–79. [Google Scholar]
- 51.International Agency for Research on Cancer (IARC) 1999. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 71. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide summary of data reported and evaluation [Internet]http://monographs.iarc.fr/ENG/Monographs/vol71/volume71.pdf [cited 2012 Mar 8]. Available from: [PMC free article] [PubMed] [Google Scholar]
- 52.Hawkins N.C., Evans J.E. Subjective estimation of toluene exposures: a calibration study of industrial hygienists. Appl Ind Hyg. 1989;4:61–68. [Google Scholar]


