Graphical abstract
Keywords: WIPO Re:Search, Capacity-building experience, Cross-sector collaboration, Anthelmintic
Highlights
-
•
WIPO Re:Search leverages pharmaceutical assets to advance anthelmintic research.
-
•
BIO Ventures for Global Health (BVGH) facilitates collaborations and capacity building.
-
•
Novartis scientists trained a Cameroonian researcher on advanced biochemistry skills.
-
•
Researchers from Canada and Cameroon partnered to discover onchocerciasis drugs.
-
•
Natural products were screened against schistosomes and soil-transmitted helminths.
Abstract
Neglected tropical diseases (NTDs), malaria, and tuberculosis have a devastating effect on an estimated 1.6 billion people worldwide. The World Intellectual Property Organization (WIPO) Re:Search consortium accelerates the development of new drugs, vaccines, and diagnostics for these diseases by connecting the assets and resources of pharmaceutical companies, such as compound libraries and expertise, to academic or nonprofit researchers with novel product discovery or development ideas. As the WIPO Re:Search Partnership Hub Administrator, BIO Ventures for Global Health (BVGH) fields requests from researchers, identifies Member organizations able to fulfill these requests, and helps forge mutually beneficial collaborations. Since its inception in October 2011, WIPO Re:Search membership has expanded to more than 90 institutions, including leading pharmaceutical companies, universities, nonprofit research institutions, and product development partnerships from around the world. To date, WIPO Re:Search has facilitated over 70 research agreements between Consortium Members, including 11 collaborations focused on anthelmintic drug discovery.
1. Introduction
Interest in, and support for, global health have surged in the past 20 years (Institute for Health Metrics and Evaluation, 2012). The public, private, and civil sectors have recognized the burden that diseases of poverty have on the global economy, as well as the economic growth that could be realized by alleviating the impact that these diseases exact on individuals and communities in low- and middle-income countries (World Bank, 1993). In 2010, the World Health Organization profiled 17 diseases it deemed to be “neglected” – diseases that disproportionately affect the poor, cause significant morbidity and mortality, and lack safe, effective, and affordable drugs, vaccines, and diagnostics (Savioli and Daumerie, 2010; Smith and Taylor, 2013). These neglected tropical diseases (NTDs) infect over a billion individuals worldwide and negatively affect the lives of many more (Hotez et al., 2014). A variety of approaches have been employed to combat these diseases, including education, vector control, sanitation and hygiene, behavioral change, and mass drug administration (MDA) programs. In addition, several initiatives have been established to stimulate and support the development of new products for these 17 diseases. These initiatives include product development partnerships (PDPs), such as the Drugs for Neglected Diseases initiative (DNDi) and the Sabin Vaccine Institute, as well as the Uniting to Combat NTDs coalition and the WIPO Re:Search consortium.
The World Intellectual Property Organization (WIPO), a UN agency, was established in 1967 to promote the use of an international intellectual property (IP) system to stimulate innovation and creativity. Its Global Challenges division focuses specifically on exploiting IP to address some of the world’s toughest challenges, including public health (Dent et al., 2013). In 2011, WIPO, BIO Ventures for Global Health (BVGH), and eight leading pharmaceutical companies (Alnylam, AstraZeneca, Eisai, GlaxoSmithKline, MSD [Merck], Novartis, Pfizer, and Sanofi) established the WIPO Re:Search consortium to facilitate access to the biopharmaceutical industry’s IP assets. The Consortium’s objective is to leverage the IP and product development expertise of biopharmaceutical companies to accelerate the development of new drugs, vaccines, and diagnostics for NTDs, malaria, and tuberculosis. Through WIPO Re:Search, biopharmaceutical companies share their IP assets, such as compounds/compound libraries and data, as well as knowledge and expertise, with academic and nonprofit neglected disease researchers conducting novel product development projects.
Eligible research organizations join WIPO Re:Search as User, Provider, and/or Supporter Members. Provider Members, such as the founding biopharmaceutical companies, share their IP assets with User Members. The User Members, such as universities and nonprofit research institutes, utilize these IP assets to further their discovery and development of products for the Consortium’s core diseases. Supporter Members join WIPO Re:Search to demonstrate their support of the Consortium’s activities and objectives. Prior to joining, all Members must agree to abide by the WIPO Re:Search Guiding Principles (Box 1). These Principles ensure that products developed through WIPO Re:Search will be accessible to those who need the products the most. In the three years since its launch, the Consortium’s membership has expanded to over 90 for-profit, academic, nonprofit, and government research organizations. These institutes span the globe – representing 24 countries from six continents, including 25 research centers located in developing countries.
Box 1. The WIPO Re:Search Guiding Principles seek to ensure that all products developed through Consortium partnerships will be accessible to the individuals most affected by NTDs, malaria, and tuberculosis. The Guiding Principles are not legally binding. Each WIPO Re:Search partnership is governed by its specific agreement, however it is expected that these agreements embody the spirit of the Guiding Principles. The LDCs are the 49 countries identified and defined by the United Nations Office of the High Representative for Least Developed Countries, Landblocked Developing Countries and the Small Island Developing States (UN-OHRLLS) as of November 2010 (UN-OHRLLS, 2014).
WIPO Re:Search Guiding Principles:
-
•
Members will provide a royalty-free license for any product developed through WIPO Re:Search that is used and sold in the least developed countries (LDCs).
-
•
Members will consider the issue of access and affordability to these products for all developing countries, including those that do not qualify as LDCs.
-
•
Members will provide royalty-free licenses for NTD, malaria, and tuberculosis research and development.
-
•
IP asset users will retain ownership of any new IP developed, but are encouraged to make new inventions available to other Members of WIPO Re:Search.
As the Secretariat of WIPO Re:Search, WIPO is responsible for managing the WIPO Re:Search Database – an online database of IP assets contributed by Members; coordinating regular communication between WIPO, BVGH, and Members; and hosting the annual Member meeting. BVGH is the Partnership Hub Administrator and is responsible for facilitating connections and collaborations between Members, recruiting new Members, and communicating the Consortium’s activities and achievements. Established in 2004 by the Biotechnology Industry Organization (BIO), BVGH’s mission is to engage biopharmaceutical companies in global health initiatives. BVGH leverages its relationship with biopharmaceutical companies and its background in global health to facilitate meaningful WIPO Re:Search collaborations that accelerate product development projects.
WIPO Re:Search membership is free to all non-profit, academic, and government research organizations; for-profit Members provide annual financial sponsorship to the Consortium. This sponsorship supports BVGH’s partnering activities. Scientists participating in a WIPO Re:Search partnership are not only responsible for performing the research and development activities but are also responsible for securing funding to support these activities. Pharmaceutical company partners have assisted in securing research grants by providing their academic partners with letters of support for inclusion in grant applications. BVGH also assists the researchers by providing all Members with access to its Funders Database, a comprehensive compilation of funding awards relevant to neglected infectious disease researchers.
WIPO Re:Search has expanded past its original vision of facilitating product development collaborations between pharmaceutical companies and academic researchers. Indeed, BVGH has facilitated agreements between pharmaceutical companies and biotech startups, as well as collaborations between two academic institutions. Furthermore, to assist in the development of product development skills in the developing world, WIPO and BVGH have established several training opportunities for researchers from African Member institutions. This article presents several examples of collaborative research projects and short-term capacity-building experiences between WIPO Re:Search Members that were facilitated by BVGH.
2. WIPO Re:Search collaborations
Through its role as the WIPO Re:Search Partnership Hub Administrator, BVGH is tasked with establishing innovative research partnerships between Members. BVGH studies the scientific literature published by Members’ scientists, identifies areas of potential collaboration, and contacts the researchers to suggest partnership ideas. This proactive partnering approach has proven to be highly successful, resulting in over 70 partnerships across 15 diseases (Fig. 1). Eleven of these collaborations involve projects focused on developing new anthelmintic drugs (Fig. 2). The following describe three such WIPO Re:Search collaborations that were established to accelerate drug discovery for schistosomiasis, soil-transmitted helminthiases, and onchocerciasis.
Fig. 1.
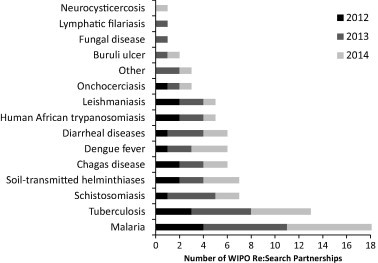
WIPO Re:Search agreements by disease focus and calendar year established. Since 2011, over 70 partnerships, representing one or more diseases, have been finalized. Adapted with permission from Ramamoorthi et al. (2014). Copyright 2014 American Chemical Society.
Fig. 2.
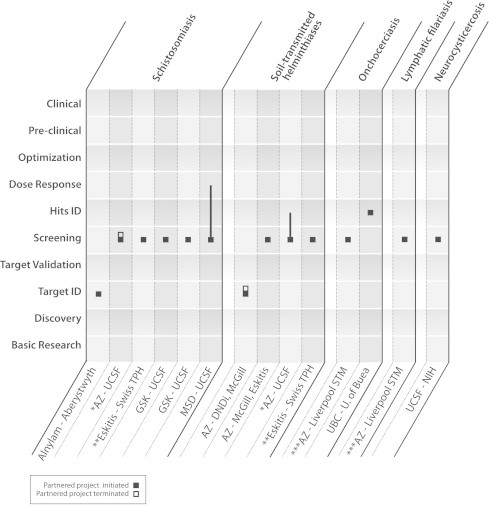
Location of WIPO Re:Search anthelmintic drug development partnerships within the product development pipeline. The majority of partnerships facilitated to date involve the sharing of compounds or compound libraries to be screened against the causative agents of schistosomiasis, soil-transmitted helminthiases, onchocerciasis, lymphatic filariasis, and neurocysticercosis. Partners mentioned in this figure include Aberystwyth University, Alnylam Pharmaceuticals, AstraZeneca (AZ), Drugs for Neglected Diseases initiative (DNDi), Eskitis Institute at Griffith University, GlaxoSmithKline (GSK), Liverpool School of Tropical Medicine (Liverpool STM), McGill University, MSD (known as Merck in the US and Canada), US National Institutes of Health (NIH), Swiss Tropical and Public Health Institute (Swiss TPH), University of British Columbia (UBC), University of California, San Francisco (UCSF), and University of Buea. Capacity-building partnerships are not included in the figure. Asterisks (∗, ∗∗, ∗∗∗) represent partnerships focused on multiple diseases.
Schistosomiasis (also known as bilharzia or snail fever) affects over 249 million individuals worldwide (World Health Organization, 2014a), predominantly in tropical and sub-tropical regions. Symptoms associated with schistosomiasis include pain, diarrhea, weakness, and hepatosplenomegaly, as well as anemia and stunting in children (World Health Organization, 2014a). Praziquantel is the only widely-available and WHO-recommended drug for treatment of the disease. This drug is thought to antagonize schistosome calcium ion channels, leading to paralysis of adult worms’ muscles (Doenhoff et al., 2008) – however this has not been demonstrated conclusively. Complicating its use, praziquantel has only limited efficacy against immature schistosome worms (Pica-Mattoccia and Cioli, 2004; Botros et al., 2005). A pediatric formulation of praziquantel is not yet available, however an international public–private partnership, the Pediatric Praziquantel Consortium, is reformulating the drug, or its active enantiomer, for use in young children (TI Pharma, 2014). With the substantial number of infections occurring annually, including in children aged ⩽5 years, and the prospect that over-reliance on a single drug will lead to widespread resistance and an inability to treat the disease, the need for a second, more broadly-acting drug that can further reduce schistosomiasis morbidity in any age group is required.
To address this, researchers at the Swiss Tropical and Public Health Institute (Swiss TPH) exploited nature, specifically the Eskitis Institute’s Nature Bank, to identify new schistosomiasis drugs. Based at Griffith University in Australia, the Nature Bank includes samples from marine organisms harvested from the Great Barrier Reef and temperate reefs near Tasmania, and terrestrial plant samples collected from the forests of Papua New Guinea, South East China, and Queensland, Australia. Combined, the Nature Bank has 45,000 samples from a total of >14,000 species of plants and marine organisms. In addition, 200,000 fractions and 3000 pure compounds have been produced from these species (Camp et al., 2014). BVGH facilitated an introduction between Prof. Jennifer Keiser, a faculty member at Swiss TPH, and Prof. Ronald Quinn, the Director of the Eskitis Institute, to explore a potential collaboration. A material transfer agreement (MTA) was put in place that allowed researchers at Swiss TPH to screen the Nature Bank’s extracts against schistosomes and soil-transmitted helminths. In the event that Swiss TPH identified any promising hits, the Eskitis Institute offered to provide additional support to identify and isolate the hits’ active components.
Soil-transmitted helminths (STHs), including Ascaris (roundworm), Trichuris (whipworm), Necator, and Ancylostoma genera (hookworms), are the most prevalent NTDs, resulting in 819 million, 464.6 million, and 438.9 million infections in 2010, respectively (Pullan et al., 2014). STHs are predominantly found in warm, moist climates, such as tropical and subtropical regions, and commonly coincide with areas of high poverty and poor sanitation and hygiene. Symptoms associated with STH infection include diarrhea, abdominal pain, general malaise and weakness, and in the case of hookworm infection, chronic anemia (World Health Organization, 2014b). Several drugs are currently available to treat STH infections, including albendazole, mebendazole, pyrantel pamoate, and levamisole, however none of these drugs is highly effective against all three types of helminths (Adegnika et al., 2014). There is only limited evidence of growing anthelmintic resistance in the helminths (Vercruysse et al., 2012), however, resistance in livestock has been demonstrated, further emphasizing the necessity of developing new, more broadly-acting drugs.
Caenorhabditis elegans has been utilized by scientists globally to examine mechanisms of disease, to map signaling pathways, and to elucidate cellular differentiation. The nematode is extremely well characterized and scientists possess the techniques, tools, and reagents that facilitate its facile maintenance and manipulation. As a nematode, C. elegans is related to numerous pathogenic helminths, including Ascaris, Trichuris, and hookworms, and thus may be utilized as a model organism for STH biology. Due to its ease of use, Prof. Joseph Dent and Prof. Tim Geary at McGill University have utilized C. elegans to screen potential STH drug candidates. To demonstrate the feasibility of utilizing C. elegans in a high-throughput screen (HTS), BVGH connected AstraZeneca and Prof. Dent and Prof. Geary. Following discussions, researchers from AstraZeneca and McGill collaborated within AstraZeneca’s Global HTS Centre to screen a 7000-compound diversity library against C. elegans as a proof of concept activity. The results of this study were incorporated into an application that requested funding to support the further screening of up to two million AstraZeneca compounds within AstraZeneca’s HTS facility. AstraZeneca, Prof. Dent, and Prof. Geary included the Eskitis Institute in this collaboration in order to also screen the Nature Bank’s natural product fractions against C. elegans. While the application was shortlisted, it was not selected for funding. This result was not entirely discouraging; both Prof. Dent and Prof. Geary expressed a willingness to develop a new strategy to move the project with AstraZeneca and the Eskitis Institute forward.
Onchocerciasis (river blindness), second only to trachoma as the leading cause of infectious blindness (Etya’ale, 2002; Centers for Disease Control and Prevention, 2013), is caused by the nematode worm, Onchocerca volvulus. The infection is managed in endemic areas through annual or semi-annual MDA of the broad-spectrum anthelmintic, ivermectin. Ivermectin treatment results in the hyperpolarization of Onchocerca glutamate-sensitive channels, which paralyzes the movement and pharyngeal pumping of immature worms (microfilariae) (Wolstenholme and Rogers, 2005). Movement and pharyngeal pumping are not required for adult worm (macrofilariae) survival, and as such, while ivermectin treatment results in a substantial (98%) reduction in dermal microfilariae (Basanez et al., 2008), it has a limited effect on macrofilariae (Taylor et al., 2010). In contrast, concurrent administration of the antibiotic, doxycycline, results in a reduction in female macrofilariae reproduction and eventual death of the adult worms. However, doxycycline, which targets the helminth’s essential intracellular Wolbachia bacteria, requires a four- to six-week course of daily administrations and is contraindicated in children and pregnant women (Tamarozzi et al., 2011). This limits the antibiotic’s utility in MDA programs. Furthermore, high Onchocerca microfilariae burden and Loa loa coinfection both have been associated with severe adverse events to ivermectin (Boussinesq et al., 2003; Merck, 2010; Taylor et al., 2010; Turner et al., 2010), thus limiting the regions where ivermectin can be used safely. Given the complications and regimens of existing drugs, there is a substantial need for new and effective treatments for onchocerciasis.
Researchers at the University of Buea, in Cameroon, have developed a medium-throughput phenotypic assay to screen compounds and natural product extracts against Onchocerca worms. Specifically, natural products are screened against Onchocerca ochengi microfilariae and macrofilariae isolated from cows at local abattoirs, as well as counter-screened against Loa loa microfilariae isolated from humans. This tripartite screen ensures that compounds displaying micro- and macrofilaricidal activities are inactive against the Loa loa filariae. Prof. Fidelis Cho-Ngwa identified several natural products with activity against the microfilariae and macrofilariae. To aid in the further development of these potential anti-onchocercal natural product extracts, BVGH connected Prof. Cho-Ngwa with a researcher from the University of British Columbia (UBC) in Canada. The UBC faculty member, Prof. Raymond Andersen, is an organic chemist by training and has the essential technologies, expertise, and experience needed to identify active compounds from natural product extracts. Through WIPO Re:Search, Prof. Andersen will assist with activity-guided fractionation to isolate and subsequently characterize the extracts’ active compounds.
These collaborations are just a few examples of the numerous partnerships that WIPO Re:Search Members are participating in. While these projects were developed on an ad hoc basis, driven by the interests and expertise of the researchers rather than to specifically fill a product pipeline, as evidenced by Fig. 2, the partnerships are clearly contributing to the product development pipeline. Bringing a product through preclinical, clinical, and regulatory filing activities is an inherently expensive endeavor that requires significant expertise. In the event that a WIPO Re:Search partnership results in a promising lead, BVGH will work with the participating Members to identify sources of funding as well as other Members with the expertise needed to transit the potential product through the pipeline to the market and those individuals that need it the most.
3. WIPO Re:Search capacity-building experiences
Cutting edge skills and knowledge, such as biopharmaceutical industry expertise, are essential for researchers to efficiently translate a novel finding into a new product. In addition to facilitating collaborative research projects between WIPO Re:Search Members, BVGH has also organized short-term capacity-building experiences for five African scientists at biopharmaceutical companies and leading academic institutions. These training experiences were implemented with financial support from the Australian Government Funds in Trust through WIPO. Through these training experiences, the scientists gained the skills and knowledge needed to move their research at their home institutions forward. The following are three examples of the research undertaken during these training experiences.
In addition to the symptoms of schistosomiasis mentioned above, Schistosoma haematobium infection has been associated with an increased incidence of bladder carcinomas. These carcinomas are thought to be induced by schistosome-mediated chronic irritation and inflammation of the bladder, and may be further exacerbated by bacterial coinfection. In schistosomiasis-endemic regions, such as Egypt, approximately 30% of all cancers are bladder cancers associated with schistosomiasis (Mostafa et al., 1999). While expansive epidemiological data demonstrate the connection between S. haematobium infection and bladder cancer, the histopathological effect of schistosomiasis on the bladder and the molecular mechanisms of its carcinogenesis are not fully understood (Honeycutt, 2014).
Prof. Olfat Hammam, a pathologist with expertise in schistosomiasis pathology from the Theodor Bilharz Research Institute (TBRI) in Giza, Egypt, participated in a WIPO Re:Search capacity-building experience at Stanford University. Prof. Michael Hsieh at Stanford had developed a microsurgery technique that recapitulated human schistosome bladder infections in mice (Fu et al., 2012). Through the capacity-building placement, Prof. Hammam used her experience as a pathologist to examine bladder carcinomas associated with schistosomiasis, and in partnership with Prof. Hsieh, investigated whether these carcinomas were directly caused by schistosome infection.
Female genital schistosomiasis caused by S. haematobium infection has additionally been associated with increased susceptibility to HIV infection (Feldmeier et al., 1994; Kjetland et al., 2006; Mbabazi et al., 2011). While at Stanford, Prof. Hammam also participated in the development of a murine model of female genital schistosomiasis that will aid in the exploration of the mechanisms of female genital schistosomiasis and its role in increased HIV susceptibility (Richardson et al., 2014).
Researchers at the Kwame Nkrumah University of Science and Technology (KNUST) in Kumasi, Ghana specialize in examining the potential of natural products and medicinal plants as anti-microbial and anti-parasitic treatments. In order to stimulate KNUST’s discovery of anti-parasitic compounds, Dr. Christian Agyare, a KNUST senior lecturer, participated in a WIPO Re:Search capacity-building experience at the Center for Discovery and Innovation in Parasitic Diseases (CDIPD) at the University of California, San Francisco (UCSF), in collaboration with Dr. Conor Caffrey. The training program provided Dr. Agyare with experience cultivating, maintaining, and screening compounds against numerous parasites, including the causative agents of Chagas disease, lymphatic filariasis, human African trypanosomiasis, and schistosomiasis. During the capacity-building placement, Ghanaian natural products were screened against the various parasitic organisms. Following the extraction and purification of compounds from those anti-parasitic products at KNUST, Dr. Agyare will return to UCSF to further examine their anti-parasitic activity. The skills and training acquired during the capacity-building placement will enhance the research program and education of undergraduate and post-graduate students at KNUST. Dr. Agyare and Dr. Caffrey’s long-term goal is to maintain and expand the collaborative activities between KNUST and UCSF.
BVGH also facilitated the placement of Prof. Fidelis Cho-Ngwa at Novartis’ headquarters in Basel, Switzerland. The three-month capacity-building experience provided Prof. Cho-Ngwa with the opportunity to work alongside Novartis scientists to obtain the knowledge and skills necessary to use HPLC and MS techniques to extract, purify, and identify the active compounds from natural products identified during his tripartite anti-onchocercal phenotypic screen.
Capacity-building opportunities such as these enable developing country researchers to learn the advanced laboratory techniques and product development processes essential to move their projects forward. These experiences foster international collaboration and cooperation, and increase the skills and capabilities of laboratories across the region, thus allowing these institutes and researchers to be active contributors to the global movement to eliminate the health inequalities of the developing world.
4. Conclusion
It is abundantly clear that new anthelmintic drugs are needed. Helminth infections represent a serious health and economic burden on individuals and communities living in poverty. They hinder childhood development, limit school attendance, and prevent a substantial section of the adult population from supporting their countries’ economic development (Hotez et al., 2008). Current anthelmintic regimens are burdened with incomplete coverage across all genera (Adegnika et al., 2014), and/or life cycle stages (Pica-Mattoccia and Cioli, 2004; Botros et al., 2005); limiting contraindications (Tamarozzi et al., 2011) and serious side-effects (Boussinesq et al., 2003; Taylor et al., 2010; Turner et al., 2010); long treatment dosing regimens (Tamarozzi et al., 2011); and the potential for the development of resistance (Vercruysse et al., 2012). As the Partnership Hub Administrator of WIPO Re:Search, BVGH facilitates meaningful partnerships between neglected disease researchers and biopharmaceutical companies with compounds to repurpose, data to utilize, and expertise to share. Through these partnerships, scientists are furthering their understanding of helminths and moving closer to developing new anthelmintic drugs.
In addition to the partnerships discussed in this article, BVGH is also establishing WIPO Re:Search collaborations that advance the development of helminth diagnostics. These include diagnostics that will be used during the transition from disease control to elimination, when testing before treatment becomes more important. The innovation spurred by the collaborations facilitated between pharmaceutical and biotechnology companies, academic institutions, and government organizations will prove essential to the development of products that eliminate the helminthic diseases that exact a devastating toll on those living in poverty. By doing so, these, and other, collaborations pave the way for improved health and economic growth across the globe.
Conflicts of interest
J Dent, R Ramamoorthi, and KM Graef are employees of BIO Ventures for Global Health, which receives WIPO Re:Search sponsorship funding from Alnylam, Eisai, GlaxoSmithKline, MSD, Novartis, Pfizer, and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Novartis’ sole involvement in the preparation of this article is through the aforementioned review of the text. None of the other sponsors were involved in the preparation of this manuscript.
Acknowledgements
The authors thank AstraZeneca, the Eskitis Institute, KNUST, McGill University, Novartis, Stanford University, Swiss TPH, TBRI, UBC, UCSF, and the University of Buea for their review and helpful suggestions.
Contributor Information
Roopa Ramamoorthi, Email: rramamoorthi@bvgh.org.
Jennifer Dent, Email: jdent@bvgh.org.
References
- Adegnika A.A., Zinsou J.F., Issifou S., Ateba-Ngoa U., Kassa Kassa F.R., Feugap E.N., Honkpehedji Y.J., Agobe J.D., Kenguele H.M., Massinga-Loembe M., Agnandji S.T., Mordmuller B., Ramharter M., Yazdanbakhsh M., Kremsner P., Lell B. Efficacy of single versus repeated dose albendazole to treat Ascaris lumbricoides, Trichuris trichiura and hookworm infection: a randomized controlled assessor-blinded clinical trial. Antimicrob. Agents Chemother. 2014;58:2535–2540. doi: 10.1128/AAC.01317-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez M.G., Pion S.D., Boakes E., Filipe J.A., Churcher T.S., Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect. Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- Botros S., Pica-Mattoccia L., William S., El-Lakkani N., Cioli D. Effect of praziquantel on the immature stages of Schistosoma haematobium. Int. J. Parasitol. 2005;35:1453–1457. doi: 10.1016/j.ijpara.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Boussinesq M., Gardon J., Gardon-Wendel N., Chippaux J.P. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003;2(Suppl. 1):S4. doi: 10.1186/1475-2883-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp D., Newman S., Pham N.B., Quinn R.J. Nature Bank and the Queensland Compound Library: unique international resources at the Eskitis Institute for Drug Discovery. Comb. Chem. High Throughput Screen. 2014;17:201–209. doi: 10.2174/1386207317666140109120515. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2013. Onchocerciasis. <http://www.cdc.gov/parasites/onchocerciasis/> (accessed June 20, 2014).
- Dent J., Ramamoorthi R., Graef K., Nelson L.M., Wichard J.C. WIPO Re:Search: a consortium catalyzing research and product development for neglected tropical diseases. Pharm. Pat. Anal. 2013;2:591–596. doi: 10.4155/ppa.13.49. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- Etya’ale D. Eliminating onchocerciasis as a public health problem: the beginning of the end. Br. J. Ophthalmol. 2002;86:844–846. doi: 10.1136/bjo.86.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeier H., Krantz I., Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. Int. J. STD AIDS. 1994;5:368–372. doi: 10.1177/095646249400500517. [DOI] [PubMed] [Google Scholar]
- Fu C.L., Odegaard J.I., Herbert D.R., Hsieh M.H. A novel mouse model of Schistosoma haematobium egg-induced immunopathology. PLoS Pathog. 2012;8:e1002605. doi: 10.1371/journal.ppat.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt J., Hammam O., Fu C., Hsieh M.H. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends. Parasitol. 2014;30:324–332. doi: 10.1016/j.pt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Alvarado M., Basanez M.G., Bolliger I., Bourne R., Boussinesq M., Brooker S.J., Brown A.S., Buckle G., Budke C.M., Carabin H., Coffeng L.E., Fevre E.M., Furst T., Halasa Y.A., Jasrasaria R., Johns N.E., Keiser J., King C.H., Lozano R., Murdoch M.E., O’Hanlon S., Pion S.D., Pullan R.L., Ramaiah K.D., Roberts T., Shepard D.S., Smith J.L., Stolk W.A., Undurraga E.A., Utzinger J., Wang M., Murray C.J., Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8:e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME) IHME; Seattle: 2012. Financing Global Health 2012: The End of the Golden Age? [Google Scholar]
- Kjetland E.F., Ndhlovu P.D., Gomo E., Mduluza T., Midzi N., Gwanzura L., Mason P.R., Sandvik L., Friis H., Gundersen S.G. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- Mbabazi P.S., Andan O., Fitzgerald D.W., Chitsulo L., Engels D., Downs J.A. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl. Trop. Dis. 2011;5:e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck . Merck & Co., Inc.; Whitehouse Station: 2010. Stromectol (Ivermectin) [Google Scholar]
- Mostafa M.H., Sheweita S.A., O’Connor P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit. Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi R., Graef K.M., Krattiger A., Dent J.C. WIPO Re:Search: catalyzing collaborations to accelerate product development for diseases of poverty. Chem. Rev. 2014 doi: 10.1021/cr5000656. [DOI] [PubMed] [Google Scholar]
- Richardson M.L., Fu C.L., Pennington L.F., Honeycutt J.D., Odegaard J.L., Hsieh Y.J., Hammam O., Conti S.L., Hsieh M.H. A new mouse model for female genital schistosomiasis. PLoS Negl. Trop. Dis. 2014;8:e2825. doi: 10.1371/journal.pntd.0002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L., Daumerie D. World Health Organization; Geneva: 2010. First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases. [Google Scholar]
- Smith J., Taylor E.M. MDGs and NTDs: reshaping the global health agenda. PLoS Negl. Trop. Dis. 2013;7:e2529. doi: 10.1371/journal.pntd.0002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarozzi F., Halliday A., Gentil K., Hoerauf A., Pearlman E., Taylor M.J. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin. Microbiol. Rev. 2011;24:459–468. doi: 10.1128/CMR.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., Hoerauf A., Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- TI Pharma, 2014. Pediatric Praziquantel Consortium awarded US $1.86 million GHIT grant. <http://www.tipharma.com/pharmaceutical-news.html?tx_ttnews%5Btt_news%5D=538&cHash=30a69bcbef42e365b1a39c30837076ce> (accessed Aug. 30, 2014).
- Turner J.D., Tendongfor N., Esum M., Johnston K.L., Langley R.S., Ford L., Faragher B., Specht S., Mand S., Hoerauf A., Enyong P., Wanji S., Taylor M.J. Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: a randomized controlled trial. PLoS Negl. Trop. Dis. 2010;4:e660. doi: 10.1371/journal.pntd.0000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN-OHRLLS, 2014. Least developed countries. <http://unohrlls.org/about-ldcs/> (accessed Aug. 30, 2014).
- Vercruysse J., Levecke B., Prichard R. Human soil-transmitted helminths: implications of mass drug administration. Curr. Opin. Infect. Dis. 2012;25:703–708. doi: 10.1097/QCO.0b013e328358993a. [DOI] [PubMed] [Google Scholar]
- World Bank . World Bank; 1993. World Development Report 1993: Investing in Health. World Development Report. pp. 1–342. [Google Scholar]
- World Health Organization, 2014a. Schistosomiasis Fact sheet No. 115 WHO. <http://www.who.int/mediacentre/factsheets/fs115/en/> (accessed June 20, 2014).
- World Health Organization, 2014b. Intestinal Worms. <http://www.who.int/intestinal_worms/more/en/> (accessed June 20, 2014).
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85–95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]


