Figure 1.
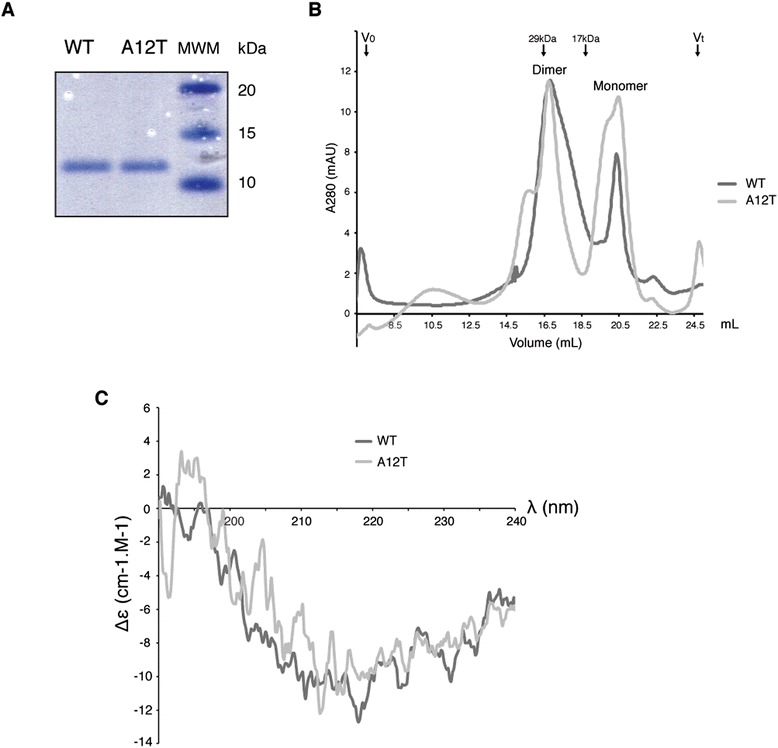
Characterization of WT and A12T Barrier to Autointegration 1 proteins. (A): Coomassie blue staining of 500 ng of recombinant wild type and A12T HexaHis-tagged BANF1 run on a Nu-Page gel. (B): Gel filtration chromatography of wild type and A12T mutant BANF1 proteins. Absorbance at 280 nm was plotted against the elution volume. Both proteins eluted in two predominant peaks, which are consistent with dimeric and monomeric BANF1. Vo indicates the void volume of the column and Vt indicates the termination volume. Arrows indicate the elution volume of the protein standards carbonic anhydrase (29 kDa) and Myoglobin (17 kDa), which were used to calibrate the column prior to BANF1 filtration. (C): A12T BANF1 secondary structure is not modified when compared to the WT. CD spectroscopy of wild-type and A12T BANF1 protein. The mean residue ellipticity Δε of the indicated protein is plotted against the wavelength, and is the results of 3 independent measurements. Δε is in cm-1.M-1.
