Figure 3.
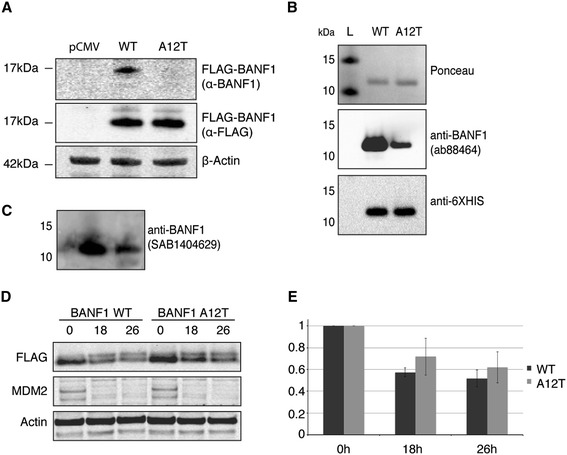
Alteration of BANF1 A12T antigenicity and stability. (A): The anti-BANF1 antibody (Abcam: ab88464) does not recognize exogenous A12T BANF1 in cell lysates. Cell extracts of U2OS cells overexpressing WT or A12T 3x FLAG-tagged BANF1 or an empty vector (pCMV-AN-3DDK) were resolved on a Nu-page gel and immunoblotted using an anti-BANF1 antibody. Membranes were stripped and immunoblotted using an anti-FLAG antibody. A β-actin antibody was used as an internal protein loading control. (B): Recombinant A12T BANF1 has a lower antigenicity than WT BANF1. 500 ng of recombinant HexaHis tagged WT and A12T BANF1 were run on a Nu-page gel and subsequently transferred to a nitrocellulose membrane. The membrane was stained using Ponceau red as a loading control, then blotted with an anti-BANF1 antibody (Abcam ab88464). After visualization, the membrane was stripped and immunoblotted using an anti HexaHis antibody. (C): Recombinant A12T BANF1 has a lower antigenicity than WT BANF1. BANF1 was visualized by blotting with an anti-BANF1 antibody (SAB1404629). (D): Wild type and A12T mutant 3x FLAG BANF1 protein stability. Cells were incubated with cycloheximide (50 μg/ml) for the indicated time periods and cell lysates harvested for western blot analysis. Protein levels were assessed using anti-FLAG antibodies to detect FLAG- tagged BANF1 and anti-actin antibodies for protein loading. MDM2 degradation is shown as a positive control for the cycloheximide treatment. (E): Quantification of (D). Band signal intensity was quantified using ImageJ and standardized against the protein level a t = 0. Error bars represent the standard deviation (SD) from at least three independent experiments.
