Figure 4.
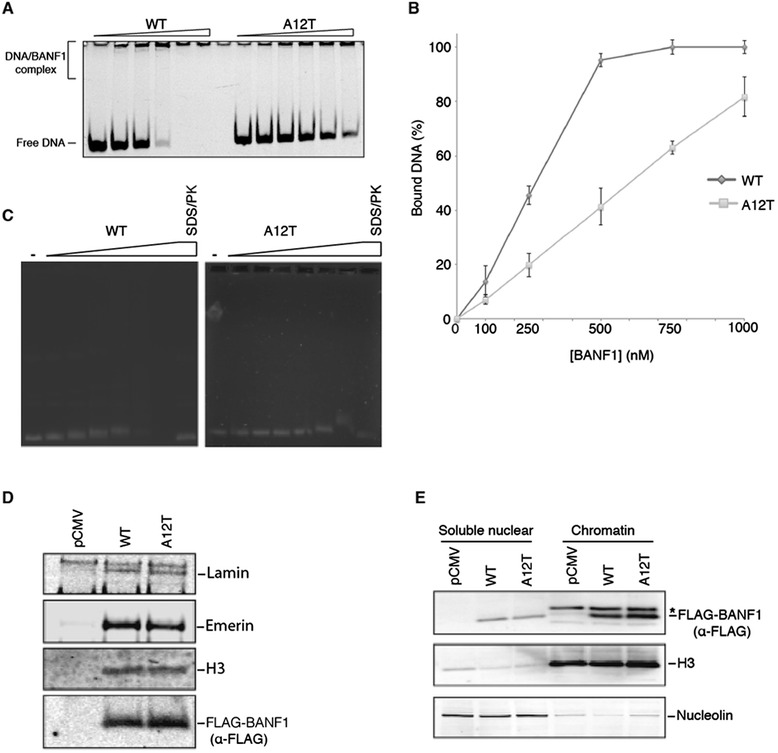
Alteration of BANF1 A12T DNA binding. (A): A12T BANF1 has a reduced affinity for short double-stranded DNA. WT and A12T BANF1 (0, 0.1, 0.25, 0.5, 0.75, 1, μM) was incubated for 30 min at 4°C with 10 nM of dsDNA that was labeled with a 5’ FAM label. The FAM label was visualized using a Starion FLA-9000 image scanner. (B): Quantification of (A). Intensity of the signal was quantified using MultiGauge software (Fujifilm). Error bars represent the standard deviation (SD) from at least three independent experiments. (C): A12T BANF1 has a reduced affinity for long double-stranded DNA. WT and A12T BANF1 (0, 0.1, 0.25, 0.5, 1, 2 and 4 μM) was incubated for 30 min at 4°C with 150 ng of double stranded DNA plasmid. The binding was visualized on an agarose gel following staining with Ethidium bromide. (D): The interaction between WT or A12T BANF1 and known partners was tested by co-immunoprecipitation. HeLa cells were transfected with the indicated vectors and exogenous protein expressed for 24 hours Total protein was then extracted and treated with Benzonase to degrade genomic DNA. M2 magnetic FLAG beads were used to immunoprecipitate 3x FLAG BANF1 and eluent probed using specific antibodies against Lamin, Emerin and Histone H3. (E): A12T BANF1 nuclear distribution is similar to that of WT BANF1. HeLa cells were transiently transfected with the indicated vectors prior to sub-cellular fractionation. Western blotting of fractions was performed using an anti-FLAG antibody (to detect exogenous BANF1), anti-H3 (chromatin fraction loading control) and anti-nucleolin (soluble nuclear loading control). *Bleed-through from antiH3 channel.
