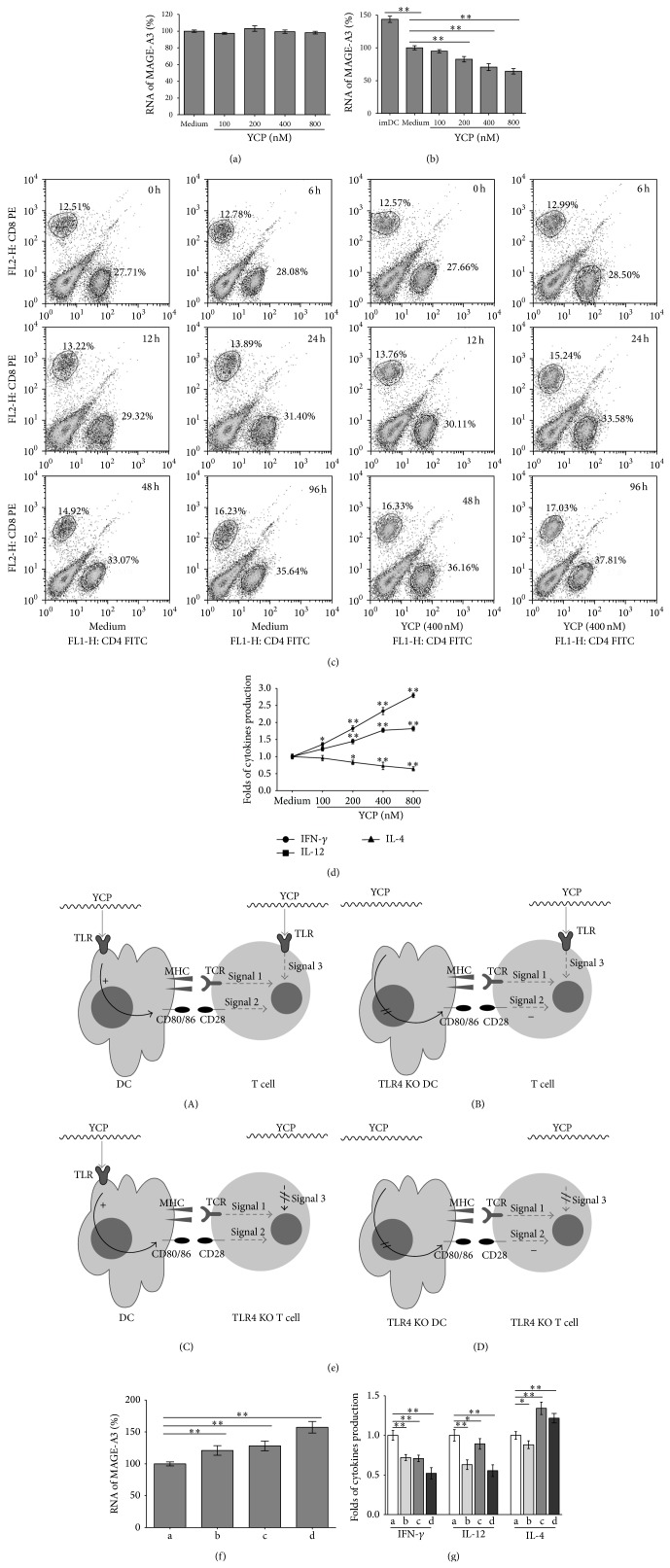
Figure 4.
The effect and mechanism of YCP on the specific immunity enhancement. (a) The target cells (B16F10 cells) stimulated by medium or YCP (100–800 nM) for 48 h directly. The number of target cells was measured by the mRNA quantitation of MAGE-A3. (b) The effector cells (T cells were cultured with mDCs at the ratio of 20 : 1 for 48 h) and target cells (B16F10 cells) were cocultured and stimulated by medium or YCP (100–800 nM) for 48 h. The number of target cells was measured by the mRNA quantitation of MAGE-A3. (c) The populations of CD4+ T cells and CD8+ T cells treated by medium or YCP (400 nM) were detected by flow cytometry during the incubation (0 h, 6 h, 12 h, 24 h, 48 h, and 96 h). (d) The expressions of IFN-γ, IL-12, and IL-4 concentration in supernatants were measured using ELISA assay after the incubation at 48 h. (e) Four cell groups were prepared to study the signal provided by YCP during in vitro specific immune responses as follows: (a) matured DCs (WT) and T cells (WT), (b) matured DCs (TLR4 KO) and T cells (WT), (c) matured DCs (WT) and T cells (TLR4 KO), and (d) matured DCs (TLR4 KO) and T cells (TLR4 KO). (f) The effector cells (T cells were cultured with mDCs at the ratio of 20 : 1 for 48 h) and target cells (B16F10 cells) were cocultured and stimulated by medium or YCP (100–800 nM) for 48 h. The number of target cells in each group was measured by the mRNA quantitation of MAGE-A3. (g) The expressions of IFN-γ, IL-12, and IL-4 concentration in supernatants were measured using ELISA assay after the incubation in each group at 48 h (n = 6, * P ≤ 0.05, ** P ≤ 0.01 by Mann-Whitney U test).