Fig. 1.
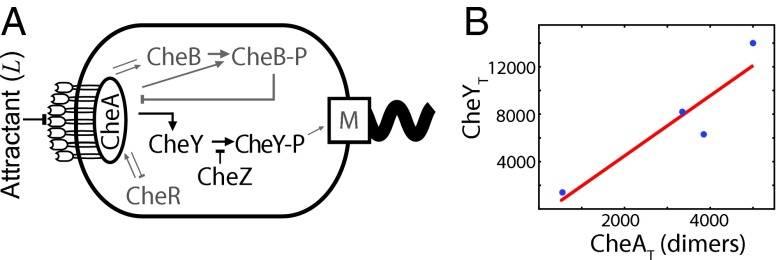
The chemotaxis network of E. coli obeys the principle of optimal resource allocation, which states that in an optimally designed system each cellular resource is equally limiting. (A) Cartoon of the sensing system. The receptor is via the adaptor protein CheW associated with the kinase CheA. This complex, coarse-grained as R in our model, can bind extracellular ligand L and activate the intracellular messenger protein CheY (x in our model) by phosphorylating it; phosphorylated CheY controls the rotation direction of the motor. Deactivation, i.e., dephosphorylation, of CheY is catalyzed by the phosphatase CheZ; the effect of CheZ is coarse-grained into the deactivation rate. The proteins CheR and CheB, which implement adaptation, have been omitted, because we are interested in the lower bound on the accuracy of sensing in static environments. (B) The principle of optimal resource allocation, Eq. 5, predicts that the number of CheY proteins, , scales linearly with the number of receptor–CheA complexes, , with a slope given by the relaxation time of the signaling network, , over the correlation time of the receptor ligand-binding state, . Plotted are data from ref. 23 for two E. coli strains under two different growth conditions; the number of CheA dimers is a proxy for the number of receptor–CheA complexes. The line is a best fit to the data, having a slope of . The resource allocation principle, Eq. 5, thus predicts that . This is on the same order of magnitude as that given by the relaxation time, (24), and correlation time , estimated from the measured receptor–ligand dissociation constant (25) and association rate (26).