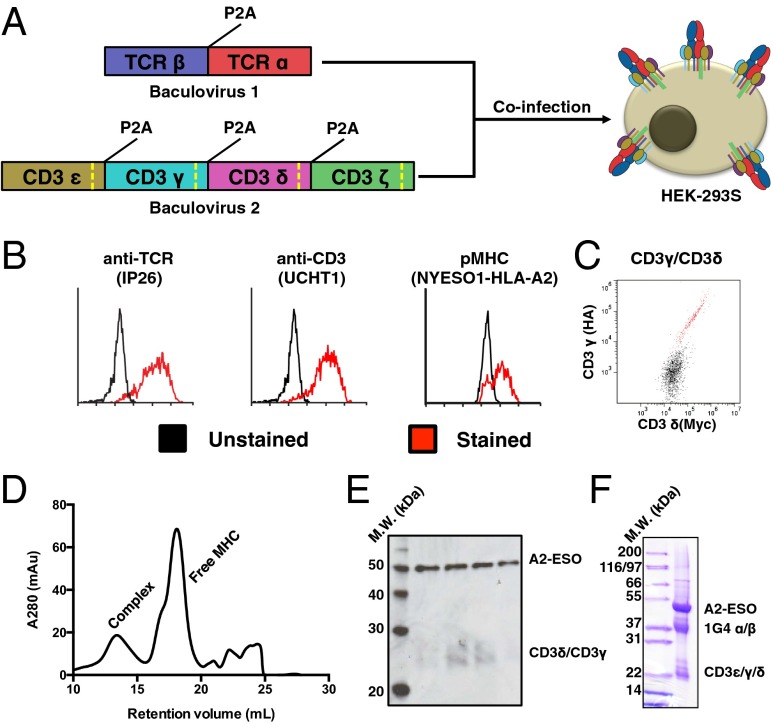
Fig. 2.
Production and characterization of full-length 1G4 TCR–CD3 complex. (A) Schematic for expression of 1G4 TCR–CD3 complex in HEK-293 cells via coinfection of baculoviruses. The baculoviruses respectively encode for TCRα/β and CD3ε/γ/δ/ζ, with each polypeptide chain separated by a viral 2A peptide (P2A). Each CD3 subunit contains a Rhinovirus 3C protease cleavage site (dashed yellow line) to remove intracellular domains after protein expression. (B) Expression of folded TCR–CD3 complex on HEK-293 cells as demonstrated by an anti-TCR antibody (Left), anti-CD3ε antibody (Center), and cognate pMHC (Right). A high-affinity TCR allows for staining of monomeric pMHC. (C) Equal staining for orthogonal epitope tags on the N termini of CD3γ and CD3γδ indicate 1:1 incorporation of CD3εγ and CD3εδ into the TCR–CD3 complex. (D) Size-exclusion chromatography for the TCR–CD3 complex bound by pMHC. (E) Western blot of size-exclusion chromatography fractions of the pMHC–TCR–CD3 complex peak shows staining for pMHC (via anti-β2m antibody) and CD3γ/δ (via anti-His antibody). The blot was simultaneously treated with both primary antibodies. (F) SDS-PAGE gel of final 1G4 TCR–CD3–ESO–A2 material showing presence of TCR, CD3, and MHC. CD3ζ is not visible because of its small size after protease cleavage (5 kDa).