Fig. 5.
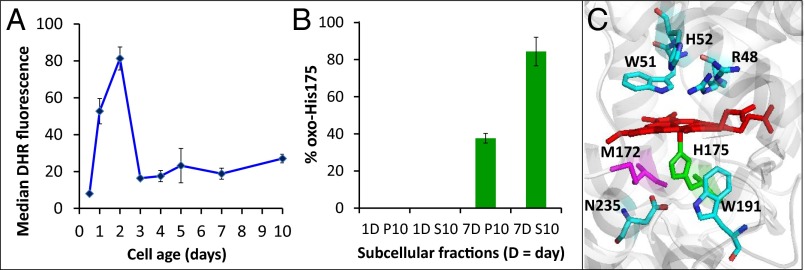
Ccp1 is oxidized at His175. (A) FACS measurements of H2O2 in wild-type cells stained with dihydrorhodamine 123 (DHR) (10). Data points are the median fluorescence per cell (in relative fluorescence units, RFU) measured for 10,000 cells per sample. (B) Percentage of oxidized His175 in mitochondrial (P10) and extramitochondrial (S10) Ccp1 from 1-d and 7-d yeast cells based on tryptic peptide peak areas in the LC-MS spectra (note that no His175 oxidation is detected in 1-d cells). See SI Appendix, Fig. S6 and SI Materials and Methods for further experimental details. (C) Ribbon diagram of the heme-binding site of Ccp1 showing the proximal Fe ligand His175 (green). Residues important in the activation of H2O2 following its binding to the vacant sixth coordination site of FeIII are also shown. This diagram was generated using PyMOL software with the coordinates from Protein Data Bank 1ZBY.