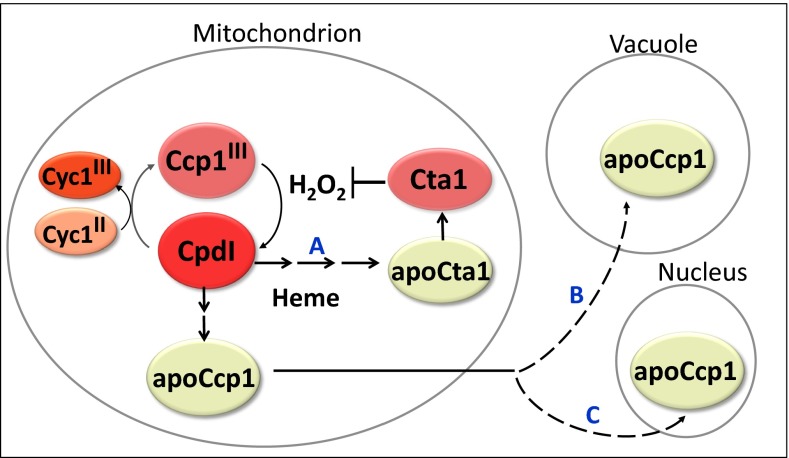
Fig. 6.
Respiration-triggered heme transfer in yeast mitochondria. H2O2 generated during mitochondrial respiration oxidizes Ccp1 to compound I (CpdI). The oxidizing equivalents from H2O2 can be reduced by Cyc1II or transferred to the peroxidase’s residues, including the proximal Fe ligand His175 to yield oxo-histidine. Formation of the latter labilizes the heme group, which is transferred either directly or via unidentified intermediate(s) to apoCta1 (path A), and the nascent Cta1 activity detoxifies H2O2. ApoCcp1 has low conformational stability and undergoes reverse translocation to the vacuole (path B) and the nucleus (path C).