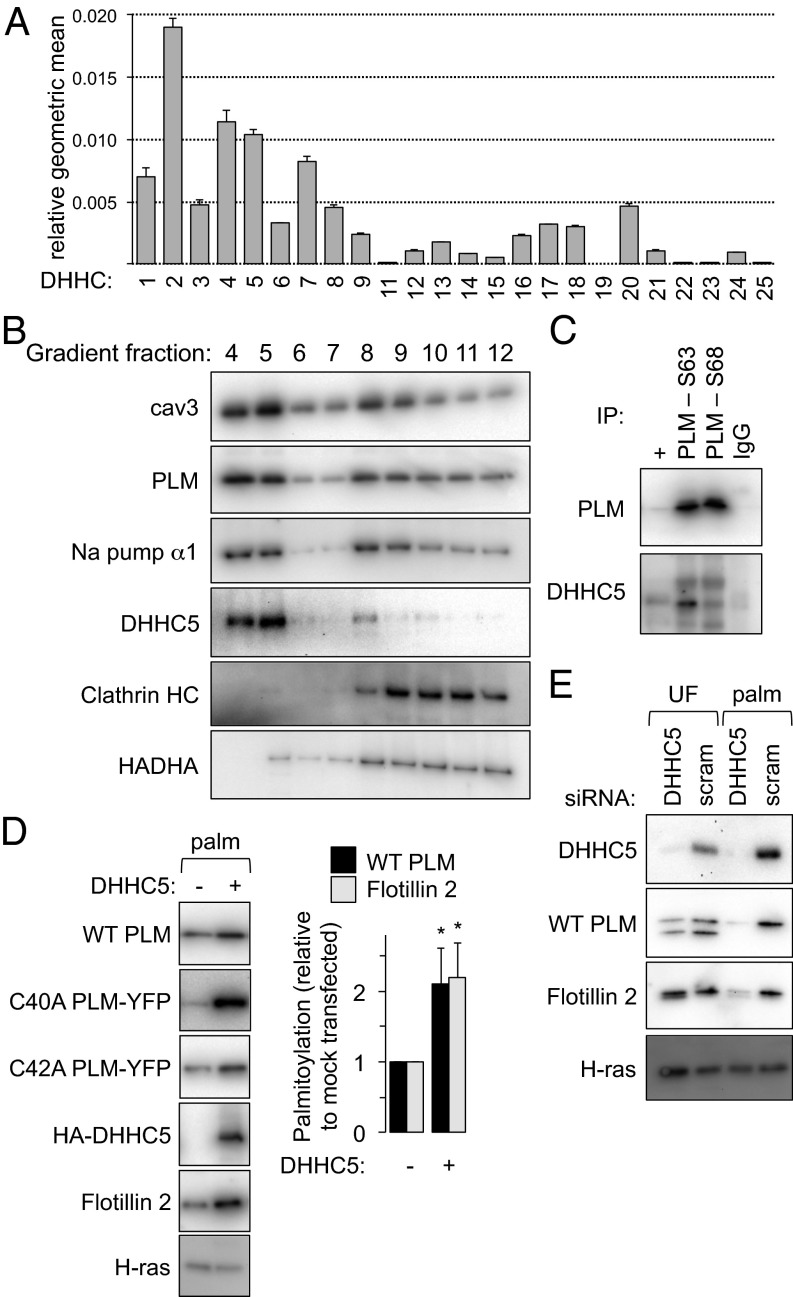
Fig. 2.
DHHC expression profile and palmitoylation of PLM. (A) The expression profile of DHHCs in cardiac muscle is expressed as the geometric mean relative to β-actin and GAPDH (n = 4). An essentially identical expression profile was obtained normalizing to β-actin only or GAPDH only. (B) DHHC5 localizes to buoyant caveolin-enriched microdomains with PLM and the Na pump α-subunit in ventricular myocytes. Sonicated ventricular homogenates were separated using a standard discontinuous sucrose gradient. Fractions were collected from the top of the gradient. Caveolin-enriched membranes are found in fractions 4 and 5, whereas bulk sarcolemmal [marker protein, clathrin heavy chain (HC)] and mitochondrial membranes [marker protein, HADHA (hydroxyacyl CoA dehydrogenase)] are exclusively found in dense membranes. (C) PLM and DHHC5 are physically associated in ventricular muscle. PLM phosphorylation states (S63 and S68) were immunoprecipitated from ventricular lysates and immunoblotted as shown. +, unfractionated ventricular lysate. (D) DHHC5 overexpression increases PLM palmitoylation in FT-293 cells stably expressing wild-type PLM, C40A PLM–YFP and C42A PLM–YFP. Flotillin 2 palmitoylation is also elevated in the same cells (palm, palmitoylated proteins purified by Acyl-RAC, *P < 0.05). (E) DHHC5 was silenced in FT-293 cells. Palmitoylation of both PLM and Flotillin 2 was significantly reduced in the absence of DHHC5. Inhibiting palmitoylation of PLM increases its degradation rate (9), so the steady-state expression of PLM was reduced when DHHC5 was silenced. UF, unfractionated cell lysate.