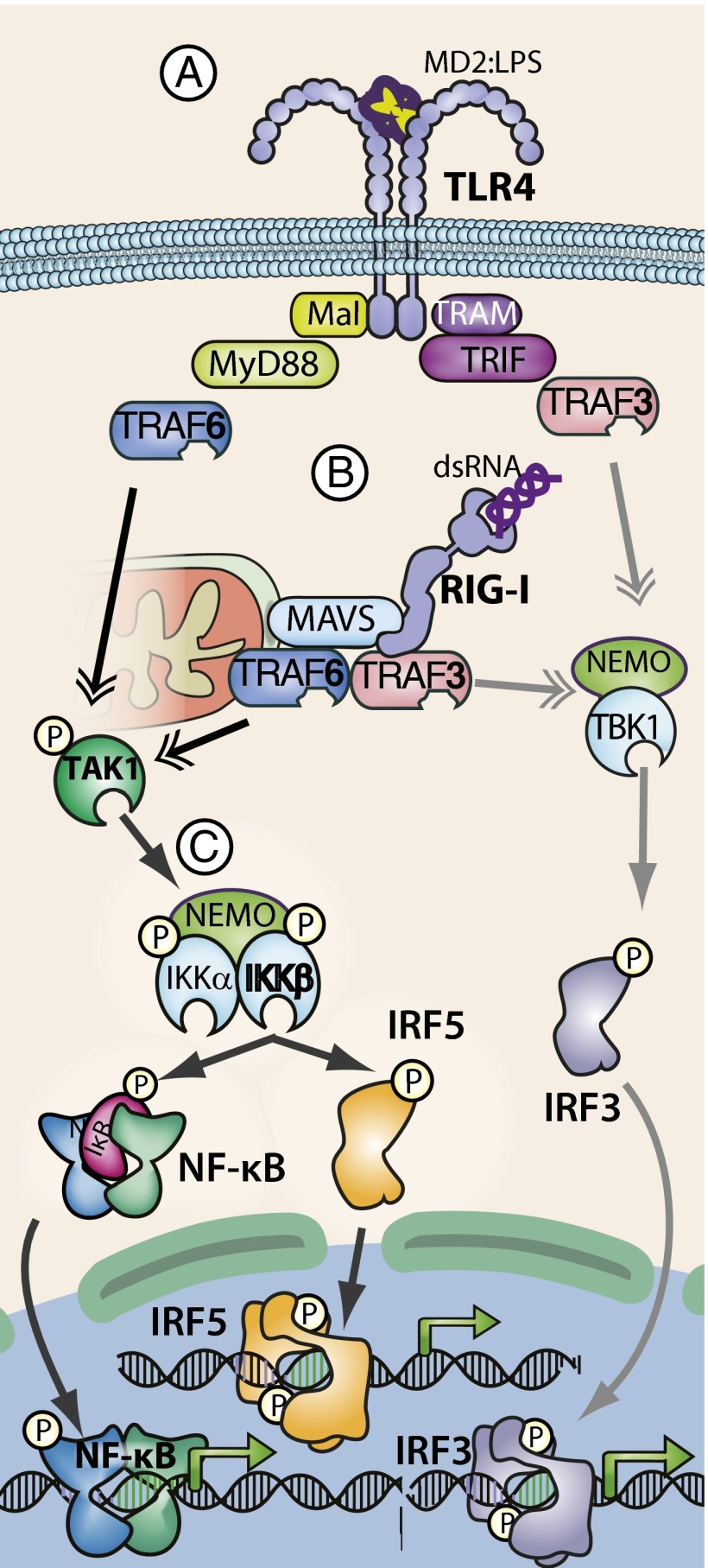
Fig. 1.
TLRs and RLRs activate IRF5 and NF-κB through IKKβ. (A) Upon binding to LPS, TLR4 undergoes dimerization and conformational changes, resulting in the recruitment of intracellular adaptor molecules. Mal/MyD88 (myeloid differentiation primary response gene 88) recruitment leads to TAK1 and IKK complex activation through a TRAF6-dependent mechanism. TRAM (TRIF-related adaptor molecule)/TRIF (TIR domain-containing adapter-inducing IFNβ) adaptor binding to MyD88 leads to TRAF3 and NEMO-dependent activation of TBK1/IKKε, which phosphorylates IRF3, leading to dimerization and IRF3-dependent gene expression. (B) Upon binding to dsRNA, RIG-I binds the mitochondria-associated adaptor protein MAVS. MAVS-dependent recruitment of TRAF6 ultimately results in activation of the TAK1 and IKK complexes. MAVS also induces TRAF3 and NEMO-dependent TBK1/IKKε activation leading the IRF3 activation. (C) TRAF6/TAK1-mediated IKK complex activation downstream of either RLR or TLR signaling results in IKKβ-dependent phosphorylation of IκBα, leading to IκBα ubiquitination and degradation and activation of NF-κB and IRF5, leading to IRF5 dimerization and nuclear localization. Activated IRF3, IRF5, and NF-κB act independently and cooperatively to regulate expression of proinflammatory cytokines, type I IFNs, and antimicrobial effectors.