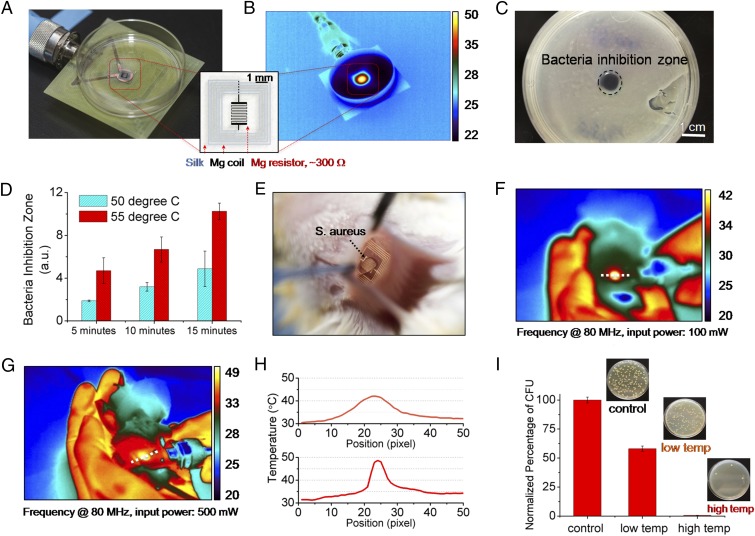
Fig. 2.
In vitro and in vivo characterization of device performance. In vitro: (A) The devices were placed underneath bacterial cultures of S. aureus grown on agar plates. (B) The device was wirelessly powered to achieve a desired temperature, monitored by an IR camera. (C) A clear zone of inhibition, after heat treatment and overnight incubation, appeared in correspondence to the area of heat treatment application. (D) Increased power and thus temperature, and duration, enhance bacterial inhibition. In vivo: (E) Photo of a device implanted in BALB/c mice. The mice were infected with a subcutaneous injection (∼5 μL) of S. aureus at the device implantation site to mimic surgical-site infections. (F and G) Two sets of 10-min heat treatments (42 and 49 °C, labeled as low temp and high temp, respectively) were carried out at a power of 100 and 500 mW after infection. (H) Measured temperature profiles corresponding to the two power levels used to remotely power the device. (I) The infected tissues were collected after 24 h and were assessed by counting the normalized number of CFU in the homogenates (n = 3) using standard plate counting methods.