Figure 3. B-Cell Aplasia.
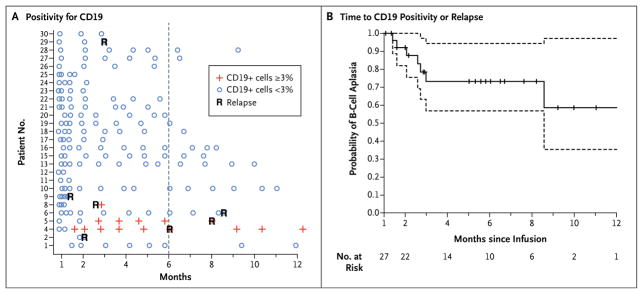
Panel A shows the results of testing to detect the percentage of CD19-positive lymphocytes in peripheral-blood samples by means of flow cytometry. Patients 1 through 25 were participants in the pediatric trial (which included children and young adults 5 to 22 years of age), and Patients 26 through 30 were participants in the adult trial (which included patients 26 to 60 years of age). Negative results were defined as less than 3% of lymphocytes that were positive for CD19. An outlier sample (Patient 16 at month 3) with 4% CD19-positive lymphocytes was discrepant with the measurements on clinical flow cytometry (<1% CD19-positive cells), bone marrow measurements at the same time point, and four subsequent monthly evaluations and was, therefore, considered to be negative. Panel B shows a Kaplan–Meier curve of the time to either CD19 positivity or relapse. Data on patients in remission were censored at the time of the last follow-up (indicated by tick marks). Dashed lines represent 95% confidence intervals. All patients required intravenous immunoglobulin replacement, and no serious infectious complications were observed as a result of B-cell aplasia; however, bronchitis (in one patient), acute otitis media (in two patients), salmonella infection (in one patient), and recurrent urinary tract infections (in one patient) were observed.
