Figure 3.
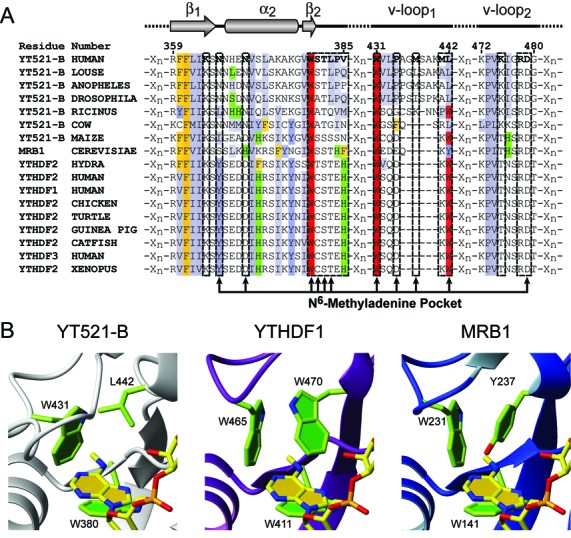
Alignment of YTH domains and homology models. (A) Clipped sequence alignment of a representative selection of YTH domain containing proteins. Regions shown include amino acids involved in RNA binding. Hydrophobic residues are colored gray, aromatic residues (F,Y,H,W) yellow, blue, green and red, respectively. Top: secondary structure representation of selected regions; first β-strand (β1), second α-helix (α2), second β-strand (β2) and the two variable loops (v-loop1 and v-loop2). Position of selected residues involved in m6A recognition are marked with arrows. (B) Homology models of the YTH domains of Homo sapiens YTHDF1 and S. cerevisiae MRB1 binding m6A. The corresponding perspective from the presented structure is shown for comparison. Representation as in (Figure 2A and C), except that the protein ribbon of YTHDF1 is in purple and the one of MRB1 in blue.
