Figure 4. Effects of divalent metal cations and pH on C. glutamicum Mca activity.
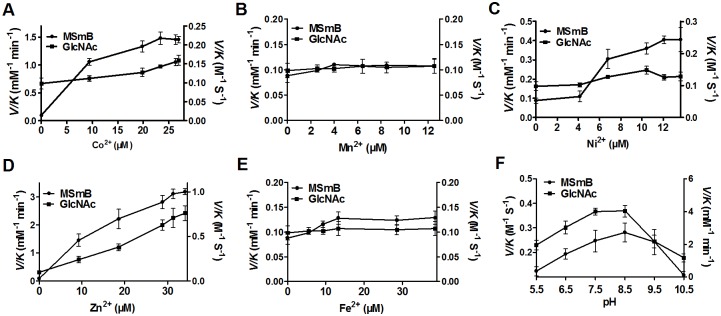
A–E. Catalytic activity of Mca in the presence of Co2+(A), Mn2+(B), Ni2+(C), Zn2+(D) and Fe2+(E), respectively, was analyzed with GlcNAc or MSmB as substrates. Apo-Mca was incubated with stoichiometric amounts of metal ions. After 30 min, the enzyme was diluted into assay buffer containing the substrate GlcNAc (5 mM) or MSmB (1 mM). The amidase activity (Left Y axis) and deacetylase activity (Right Y axis) were measured as described in “Materials and Methods”. F. Deacetylation of GlcNAc and amidase activity of MSmB by Zn2+-Mca at different pH levels. The V/K values were measured with 5 mM GlcNAc as substrate for deacetylase activity (Left Y axis) or 1 mM MSmB as substrate for amidase activity (Right Y axis) under six different pH values. pK a values of 6.5 and 9.5 were determined by fitting Equation 1 to the data (bars represent standard error of the mean).
