Abstract
Serine integrases catalyze the integration and excision of phage genomes into and out of bacterial chromosomes in a highly specific and directional manner, making these proteins powerful tools for genome engineering. In 2013, the first structure of a serine integrase-DNA complex was reported. This work revealed how the phage attP sequence is recognized by the integrase and provided important clues about how serine integrases bind to other attachment site sequences. The resulting structural models indicate that distinct spatial arrangements of integrase domains are present for each attachment site complex. Here we describe how serine integrases may exploit this site-dependent domain arrangement to regulate the direction of recombination. We also discuss how phage-encoded recombination directionality factors could change this directionality by altering the nature of inter-subunit interactions.
Keywords: serine integrase, site-specific recombination, integration, excision
Introduction
Serine integrases are members of the serine recombinase superfamily that catalyze site-specific integration and excision of phage genomes into and out of bacterial host chromosomes [1,2]. A recently proposed model for serine integrase-mediated recombination is shown in Figure 1. During viral integration, an integrase (Int) dimer binds to specific ‘attachment site’ sequences in the phage (attP) and host (attB) DNA. The Int-attP and Int-attB complexes associate to form a tetrameric intermediate, where all four substrate DNA strands are cleaved and the Int subunits become covalently coupled to their corresponding DNA half-sites. Strand exchange occurs when two Int-DNA subunits exchange positions by rotating 180° with respect to the other two subunits and the re-aligned DNA half-sites are ligated to generate new attachment sites (attL and attR) [3–5].
Figure 1.
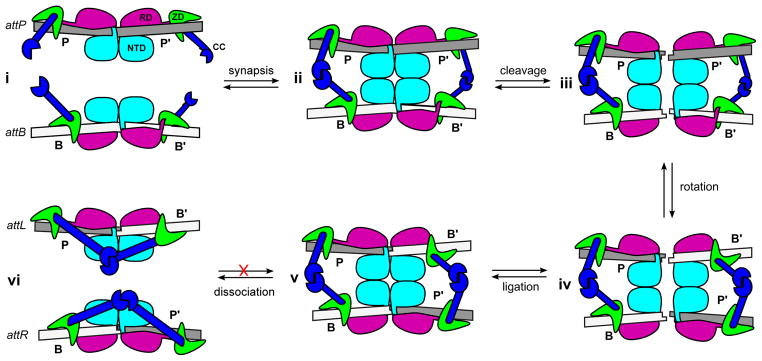
Recently proposed model of the integration reaction catalyzed by serine integrases. i) Integrase (Int) dimers bind to specific sequences in the phage (attP) and host (attB) DNA. The Int-attP and Int-attB complexes are conformationally distinct due to different positioning of a zinc ribbon domain (ZD). ii) Int-attP and Int-attB associate to form a synaptic complex that is stabilized by interactions between coiled-coil (CC) motifs. iii) The Int subunits cleave all four DNA strands at the central dinucleotide, forming 5′-phosphoserine linkages between integrase subunits and DNA half-sites (not illustrated) and generating 3′-dinucleotide overhangs. iv) The P′ and B′-linked subunits can exchange places by rotating 180° about a horizontal axis relative to the P and B-linked subunits. v) Base-pairing between the central dinucleotides promotes ligation of the DNA strands, resulting in formation of two new attachment sites, attL and attR. vi) The unique arrangement of ZDs in the attL and attR sites allows the CC motifs to form intra-molecular interactions that prevent the reaction from running efficiently in the reverse direction. NTD: N-terminal catalytic domain (cyan); RD: recombinase domain (magenta); ZD: zinc ribbon domain (green); CC: coiled-coil motif (blue).
The integration reaction is effectively unidirectional; the Int-bound attL and attR sites do not efficiently recombine to form attP and attB in the absence of a phage encoded recombination directionality factor (RDF) [1,2]. The high sequence-specificity and unidirectional nature of the integration reaction has made serine integrases attractive agents in gene therapy [6] and genomic engineering applications [1,7]. Indeed, several serine integrases have been shown to specifically and stably integrate target genes into mammalian cell lines [8,9]. Although the biochemical properties of some serine integrase systems have been studied in detail, a structural and mechanistic understanding of how these enzymes recognize their attachment sites and achieve such strong site selectivity and recombination directionality has been lacking.
Recently, the structure of the C-terminal domain (CTD) from the Listeria innocua prophage integrase (LI integrase) bound to its attP half-site was determined [10]. In addition to revealing the CTD structure and how the attP site is recognized, this work provided important clues to understand how serine integrases bind to attB sites and how the attP and attB binding modes differ. Models of Int bound to the four different attachment sites (attP, attB, attL, and attR) indicate that distinct spatial arrangements of the integrase domains are present in each case, providing a mechanistic basis for understanding why only attP x attB recombination occurs in the absence of an RDF. Here we describe how serine integrases may exploit this site-dependent arrangement of domains to regulate recombination. We also discuss how RDFs could function by altering the nature of inter-subunit interactions.
Serine integrase domain structure and attP-binding
Serine integrases are comprised of two conserved regions: a ~130 residue N-terminal catalytic domain (NTD) and a 300–500 residue C-terminal domain (CTD). The domains are connected by a long helix (αE). The NTD contains the catalytic serine residue responsible for the enzyme family name and is also involved in synapsis (association) of attachment sites, subunit rotation, and mediating strand exchange [2,3]. αE contributes substantially to the formation of the hydrophobic dimerization and tetramerization interfaces and also binds to the DNA minor groove near the center of the attachment sites [4,11,12]. The structure of a tetrameric form of the NTD from the TP901-1 integrase has been reported [13] and a two-domain φC31 integrase structure containing the NTD has been deposited in the protein data bank (PDB ID 4BQQ). As anticipated, these NTDs are highly similar in structure to those of the well-studied resolvase/invertase members of the serine recombinase family [14,15].
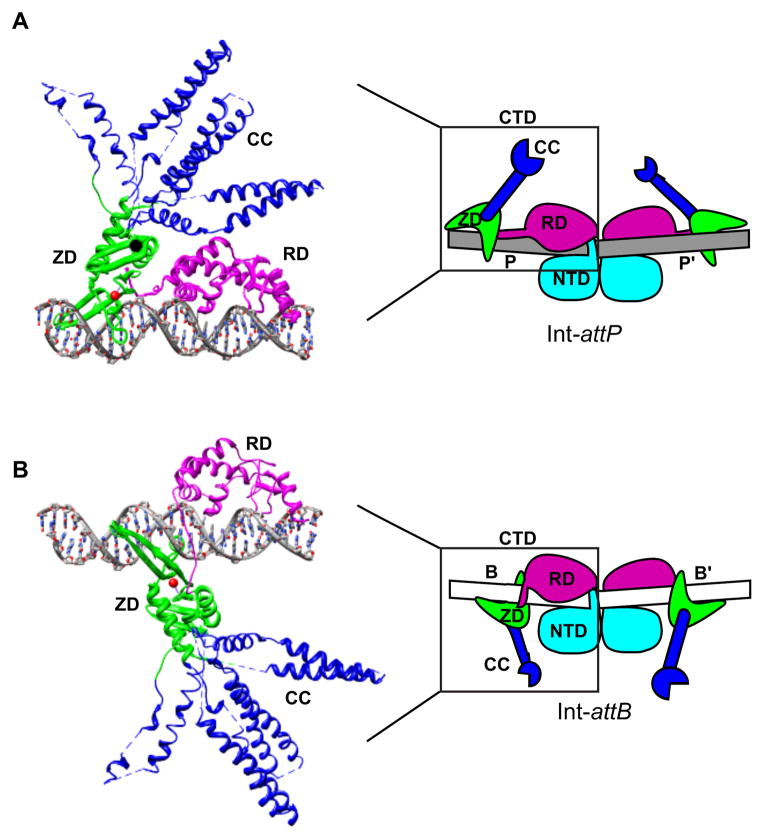
The CTD contains most of the enzyme’s DNA-binding functionality and is therefore primarily responsible for attachment-site specificity [16–20]. This region has also been implicated in mediating synapsis of attachment sites and in regulating the directionality of recombination [18,19,21]. The recently reported structure of the LI integrase CTD bound to its attP half-site has provided a framework to understand and test each of these roles [10]. As shown in Fig. 2A, the CTD is actually comprised of three structural domains: a recombinase domain (RD), a zinc-ribbon domain (ZD), and an extended coiled-coil motif (CC) which is embedded in the ZD. The RD and ZD are separated by a short, flexible linker that plays an important role in DNA-binding.
Figure 2.
Positioning of the zinc ribbon domain distinguishes attP and attB. A) Structure of an Int C-terminal domain (CTD) bound to an attP half-site. The CTD is comprised of a recombinase domain (RD; magenta) and a zinc-ribbon domain (ZD; green) separated by a flexible linker. The RD, linker, and ZD form extensive contacts with bases in the major and minor grooves along one face of the attP half-site. The ZD contains a flexible coiled-coil motif (CC; blue) that does not contact DNA and is likely involved in protein-protein interactions. The CC motifs adopt different trajectories in the four independent complexes in the crystallographic asymmetric unit; these conformers are superimposed here. A coordinated zinc ion in the ZD is shown as a red sphere. A schematic representation of an Int dimer bound to the full attP site is shown on the right, where the region corresponding to the experimental structure is boxed. B) Structural model of an Int-attB half-site complex obtained by shifting the ZD by 5-bp towards the center of the site (see text). A schematic representation of an Int dimer bound to the full attB site is shown on the right (the NTD is not included in the structural model).
The two CTDs in an Int dimer are expected to contact 50-bp of the attP site, forming extensive contacts with the major and minor grooves. The RD interacts with a stretch of 13 base-pairs adjacent to the central dinucleotide, while the ZD forms specific interactions with a 9-bp ‘ZD motif’ at the distal end of the site. The RD alone is capable of binding to DNA, but high-affinity binding is achieved only in the presence of both the RD and ZD [18,19]. The CC motif does not interact with DNA in the crystal structure and CC deletion (ΔCC) mutants bind to attP sites with the same affinity as full-length Int [10].
A reorganization of domains when Int binds to attB
Initial comparisons of attP and attB sequences revealed little overall similarity, raising the question of how the same Int dimer could bind with similar affinity to such dissimilar sequences [1,22]. However, with knowledge of the ZD and RD-binding motifs within the attP half-site, the corresponding motifs in the attB sequence could be identified for the LI integrase and a plausible model for Int binding to attB emerged [10,23]. The most important finding was that the ZD-binding motif in attB is shifted 5-bp (one half of a helical turn) towards the central dinucleotide relative to its position in attP. This explains why attB tends to be ~10-bp shorter than attP in most systems [2,23] and is supported by several sources of biochemical and genetic data [10,23]. When the attP and attB sites from the φC31, Bxb1, and several other serine integrase systems were scrutinized, a similarly shifted ZD motif could be observed and a common model of attachment site binding seemed likely for most or all serine integrases.
A model for Int-attB binding based on the LI integrase-DNA structure is shown in Fig. 2B. The RD binds to attB in a position similar to that seen in the Int-attP complex. Indeed, alignments of attP vs. attB sequences for a number of serine integrase systems reveals that the innermost 13-bp do share considerable similarity [10,23]. However, the ZD is positioned in the major groove one half of a helical turn from its location on attP, resulting in both a translation of the domain towards the center of the site and a rotation of the domain to the opposite face of the DNA helix. The flexible RD-ZD linker is able to accommodate this change in binding by reorienting in the minor groove. The alternative ZD location in the Int-attB half-sites also means that the CC-motifs adopt different positions and have altered trajectories in the two attachment sites.
A coiled-coil motif mediates synapsis and specifies directionality of recombination
Studies in the φC31 and Bxb1 integrase systems have demonstrated that recombination is regulated at the synapsis step of the pathway [18,24]. attP x attP and attB x attB recombination are both inefficient because neither the attP nor attB sites will self-associate to form stable synaptic complexes. The integrase NTDs play a major role in this inter-site association (Fig. 1) [3,25], but additional protein-protein interactions derived from the integrase CTDs have also been implicated [19,21]. In particular, a region in φC31 integrase that was identified as having a high likelihood of forming a coiled-coil was found to be involved in both promoting attP x attB integration and inhibiting attL x attR excision [21]. This region corresponds to the CC-motif in LI Int. When the LI Int CC-motif is deleted, ΔCC-Int can carry out both attP x attB and attL x attR recombination in an intra-molecular context (i.e., where synapsis is facilitated), but cannot carry out inter-molecular recombination [10]. Thus, the CC-motif is required both for integration and for regulation of directionality.
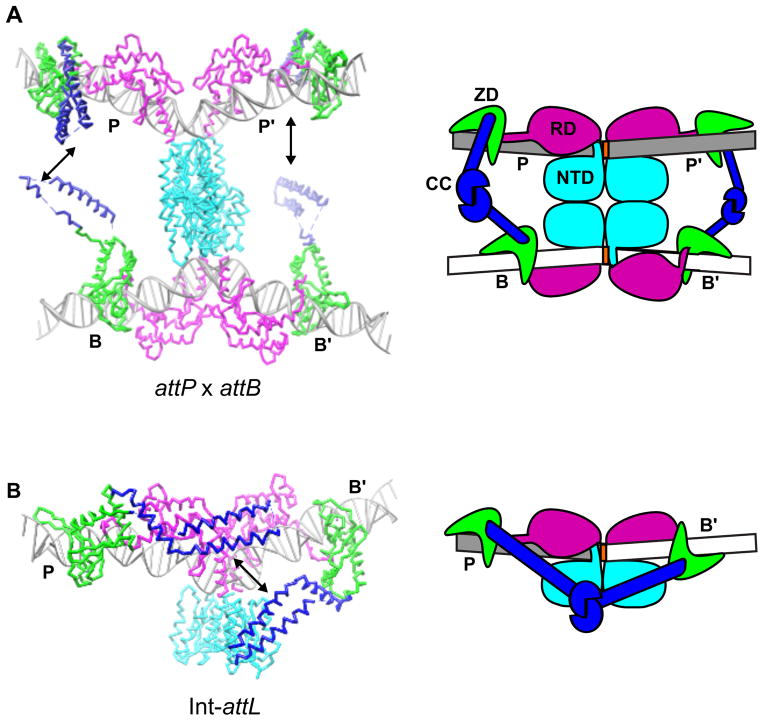
Structural models of Int-attP and Int-attB complexes suggest how the CC-motifs may function to mediate serine integrase recombination (Fig. 3A). The CC motifs in the attP x attB synaptic complex are well-positioned to form inter-subunit contacts between the P and B half-sites and between the P′ and B′ half-sites. These interactions are expected to remain intact during subunit rotation, as shown schematically in Fig. 1. In contrast, diagonal P-B′ and/or P′-B interactions would be expected to interfere with subunit rotation. Stabilizing interactions would not be present in an attP x attP synaptic complex because the CC-motifs are too far away from one another. The attB x attB complex has the opposite problem; the CC-motifs would be positioned close together and may even sterically inhibit synapsis [10].
Figure 3.
The coiled-coil motif regulates synaptic complex formation. A) Structural and schematic models of an attP x attB synaptic complex. The zinc ribbon domains of the juxtaposed half-sites (P/B and P′/B′) are oriented so that their CC-motifs can interact and stabilize the attP x attB complex without inhibiting subunit rotation. B) Structural and schematic models of the Int-attL complex. Whereas the CC-motifs are positioned on opposite faces of the DNA in the Int-attP and Int-attB complexes and would not be expected to interact with one another (see Fig. 2), the CC motifs are positioned on the same face of the complex in Int-attL and could readily interact (black arrow). The same argument can be made for the closely related Int-attR complex. Thus, intra-molecular interactions between coiled-coils in the Int-attL and Int-attR complexes may explain why excision is inhibited in the absence of an RDF protein. Different experimentally observed CC-conformers are shown in A) vs. B). Integrase domains are colored as in Fig. 1.
Amino acid substitutions in the CC motif region of ϕC31 integrase have been identified with a range of properties [21]. Some inhibit attP x attB recombination and others stimulate attL x attR recombination. Several sources of data have also suggested that the φC31 integrase CC-motifs can directly interact with one another, strengthening the idea that such interactions could stabilize synaptic complexes during recombination [19,21].
In the absence of a RDF, stable attL x attR synaptic complexes do not form [2,18,24]. This would appear to present a conundrum, since the same inter-subunit interactions that facilitate attP x attB synapsis and recombination are presumably present in the resulting attL x attR product complex (see Fig. 1). A potential solution to this puzzle is shown in Fig. 3B. The attL and attR sites are both comprised of one half-site from attP and one from attB. These hybrid sites are unique in that the ZDs are positioned on the same face of the DNA and are close enough that the attached CC-motifs could form intra-molecular interactions. We have suggested that attL and attR form auto-inhibited complexes upon their formation, preventing efficient reversal of the integration reaction as shown schematically in Fig. 1 [10]. It is important to note that these P-B′ and P′-B interactions would be expected to block strand rotation even if transient synaptic complexes were to form, perhaps explaining at least in part why the normally intra-molecular excision reaction does not occur in the absence of an RDF protein.
How might the RDF switch work?
RDFs are phage encoded proteins expressed during the lytic phase of growth [26]. In the absence of the RDF, CC-mediated interactions most likely determine which Int:att complexes are permitted to recombine (Fig. 1). In most serine integrase systems, this appears to be limited to attP x attB integration. However, in the presence of an RDF, the integration reaction is inhibited and attL x attR excision is stimulated [27–29]. How RDFs accomplish this switch in directionality is not yet understood.
The RDFs associated with five bacteriophage integrases have been identified [27–31]. While most of these RDFs share little sequence similarity and vary in size, biochemical studies suggest that they may function in a similar manner. The RDFs from bacteriophages Bxb1 (gp47) and φC31 (gp3) do not bind to attachment site DNA [28,29]. This behavior contrasts that observed for the Xis protein in the unrelated phage λ system, which binds to attachment site DNA to stimulate excisive recombination [32,33]. Instead, the serine integrase RDFs bind to the Int protein and alter the preferred direction of recombination via a different mechanism [28,29]. The Bxb1 and φC31 RDFs have also been shown to inhibit formation of integrative attP x attB synaptic complexes while stimulating formation of excisive attL x attR complexes, leading to the conclusion that synaptic complex formation is the step that is regulated by RDFs [28,29]. Similar findings have been recently reported for the bacteriophage φBT1 RDF [31].
In light of the structural framework that is now available for understanding serine integrase function, a plausible model for the mechanism of RDF switching can be considered (Fig. 4). In this model, the RDF binds to the Int CTD in a manner that disrupts the auto-inhibitory interactions present on Int-attL and Int-attR, allowing them to form synaptic complexes. It is not obvious how the same RDF-CC interactions would inhibit attP x attB synapsis. One possibility is that the RDF exploits the unique positioning of ZDs on the attB site and promotes interactions between CC motifs on that site. Such interactions have already been inferred in experiments with the φC31 integrase [10,19] and could be used in this context to reduce the efficiency of synapsis. An alternative explanation is that synaptic CC-mediated interactions are inhibited in both integrative and excisive recombination but the RDF provides compensatory interactions that are specific to the attL x attR synaptic complex. Oligomerization of the RDF may also play an important role in regulation of recombination in some systems, since the φBT1 RDF appears to form oligomers based on cross-linking experiments [31].
Figure 4.
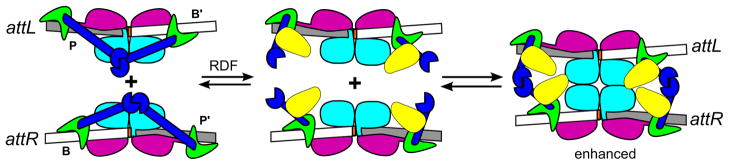
A plausible model for recombination directionality factor (RDF)-stimulated excision. The RDFs (yellow) could bind to the Int CC motifs and disrupt the intra-molecular interactions responsible for inhibiting attL x attR recombination. The RDFs may also interact with additional integrase domains and in some systems may interact with one another. Integrase domains are colored as in Fig. 1.
The model shown in Figure 4 explains several experimental observations. First, weaker apparent binding of the RDF to Int-attL and Int-attR relative to Int-attP and Int-attB is expected because of the intra-molecular competition for CC-CC interactions that would be present in the former complexes. This appears to be the case for both Bxb1 gp47 and φC31 gp3 [28,29]. Second, the RDFs could remain bound to Int in both excisive (stimulated) and integrative (weakened) synaptic complexes, which have been observed in the φC31 system [29]. Although no direct evidence currently exists for this model, the basic ideas are closely related to those already discussed in the absence of structural data [28,29,34] and are readily testable using the architectural framework that now exists to guide experiments.
Future Directions
New structural models of serine integrase-DNA complexes, combined with single-molecule biophysical investigations [35,36] as well as biochemical and genetic data from a number of laboratories [2,23], have provided a mechanistic framework for understanding how these systems function. However, there are still many gaps in our understanding on the molecular level. An experimental structure of a synaptic complex would reveal important details of the inter-subunit interactions responsible for stabilizing this important intermediate on the reaction pathway, and an Int-attL or Int-attR structure would provide important insights into the nature of the auto-inhibitory interactions that are likely to form in the products of integration. Nothing is currently known about RDF structure or the RDF-Int interaction interface for any system, making structural models of RDF-Int or RDF-Int-DNA complexes important future goals. As has been the case for many other areas, structural biology will likely play a major role in advancing our mechanistic understanding of these fascinating systems.
Highlights.
A serine integrase-DNA complex structure provides the basis for site selectivity.
A coiled-coil motif influences synaptic efficiency and promotes attP x attB recombination.
A recombination directionality factor stimulates excision and inhibits integration.
The RDF may bind the coiled-coil motif, altering site-dependent synapsis efficiency.
Abbreviations
- Int
serine integrase
- RD
recombinase domain
- ZD
zinc ribbon domain
- CC
coiled-coil
- RDF
recombination directionality factor
References
- 1.Groth AC, Calos MP. Phage integrases: biology and applications. J Mol Biol. 2004;335:667–78. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 2*.Smith MCM, Brown WRA, McEwan AR, Rowley PA. Site-specific recombination by phiC31 integrase and other large serine recombinases. Biochem Soc Trans. 2010;38:388–94. doi: 10.1042/BST0380388. A recent review of serine integrases that covers mechanistic studies through 2010. [DOI] [PubMed] [Google Scholar]
- 3.Grindley N, Whiteson K, Rice P. Mechanisms of site-specific recombination. Annu Rev Biochem. 2006;75:567–605. doi: 10.1146/annurev.biochem.73.011303.073908. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Kamtekar S, Xiong Y, Sarkis GJ, Grindley NDF, Steitz TA. Structure of a synaptic gammadelta resolvase tetramer covalently linked to two cleaved DNAs. Sci 80- 2005;309:1210–5. doi: 10.1126/science.1112064. [DOI] [PubMed] [Google Scholar]
- 5.Ritacco CJ, Kamtekar S, Wang J, Steitz TA. Crystal structure of an intermediate of rotating dimers within the synaptic tetramer of the G-segment invertase. Nucleic Acids Res. 2013;41:2673–2682. doi: 10.1093/nar/gks1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez CL, Calos MP. Therapeutic applications of the ΦC31 integrase system. Curr Gene Ther. 2011;11:375–81. doi: 10.2174/156652311797415818. [DOI] [PubMed] [Google Scholar]
- 7.Brown WRA, Lee NCO, Xu Z, Smith MCM. Serine recombinases as tools for genome engineering. Methods. 2011;53:372–9. doi: 10.1016/j.ymeth.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci U S. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keravala A, Groth AC, Jarrahian S, Thyagarajan B, Hoyt JJ, Kirby PJ, Calos MP. A diversity of serine phage integrases mediate site-specific recombination in mammalian cells. Mol Genet Genomics. 2006;276:135–46. doi: 10.1007/s00438-006-0129-5. [DOI] [PubMed] [Google Scholar]
- 10**.Rutherford K, Yuan P, Perry K, Sharp R, Van Duyne G. Attachment site recognition and regulation of directionality by the serine integrases. Nuc Acids Res. 2013;41:8341–56. doi: 10.1093/nar/gkt580. The first experimental structural data for a serine integrase CTD are reported. This work describes the CTD structure, CTD-attP recognition, and proposed models for integrase bound to attB, attL, and attR. These models lead to a plausible mechanism for coiled-coil mediated regulation of synapsis by serine integrases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W, Steitz T. Crystal structure of the site-specific recombinase gamma delta resolvase complexed with a 34 bp cleavage site. Cell. 1995;82:193–207. doi: 10.1016/0092-8674(95)90307-0. [DOI] [PubMed] [Google Scholar]
- 12.Keenholtz RA, Rowland S-J, Boocock MR, Stark WM, Rice PA. Structural basis for catalytic activation of a serine recombinase. Structure. 2011;19:799–809. doi: 10.1016/j.str.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan P, Gupta K, Van Duyne GD. Tetrameric structure of a serine integrase catalytic domain. Structure. 2008;16:1275–86. doi: 10.1016/j.str.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson MR, Freemont PS, Rice PA, Goldman A, Hatfull GF, Grindley ND, Steitz TA. The crystal structure of the catalytic domain of the site-specific recombination enzyme gamma delta resolvase at 2. 7 A resolution. Cell. 1990;63:1323–1329. doi: 10.1016/0092-8674(90)90427-g. [DOI] [PubMed] [Google Scholar]
- 15.Mouw KW, Rowland S-J, Gajjar MM, Boocock MR, Stark WM, Rice PA. Architecture of a serine recombinase-DNA regulatory complex. Mol Cell. 2008;30:145–55. doi: 10.1016/j.molcel.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Singh S, Ghosh P, Hatfull GF. Attachment site selection and identity in Bxb1 serine integrase-mediated site-specific recombination. PLoS Genet. 2013;9:e1003490. doi: 10.1371/journal.pgen.1003490. Biochemical study examining the importance of residues in the Bxb1 attP site. This work demonstrates that attP can be converted to attB by changing residues in the regions corresponding to the zinc ribbon-binding motifs of the two sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Mandali S, Dhar G, Avliyakulov NK, Haykinson MJ, Johnson RC. The site-specific integration reaction of Listeria phage A118 integrase, a serine recombinase. Mob DNA. 2013;4:2. doi: 10.1186/1759-8753-4-2. The first biochemical studies described for the bacteriophage A118 integrase, a close relative of the LI integrase used for structural studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh P, Pannunzio NR, Hatfull GF. Synapsis in phage Bxb1 integration: selection mechanism for the correct pair of recombination sites. J Mol Biol. 2005;349:331–48. doi: 10.1016/j.jmb.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 19.McEwan AR, Rowley PA, Smith MCM. DNA binding and synapsis by the large C-terminal domain of phiC31 integrase. Nucleic Acids Res. 2009;37:4764–73. doi: 10.1093/nar/gkp485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta M, Till R, Smith MCM. Sequences in attB that affect the ability of phiC31 integrase to synapse and to activate DNA cleavage. Nucleic Acids Res. 2007;35:3407–19. doi: 10.1093/nar/gkm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowley PA, Smith MCA, Younger E, Smith MCM. A motif in the C-terminal domain of phiC31 integrase controls the directionality of recombination. Nucleic Acids Res. 2008;36:3879–91. doi: 10.1093/nar/gkn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith MCM, Thorpe HM. Diversity in the serine recombinases. Mol Microbiol. 2002;44:299–307. doi: 10.1046/j.1365-2958.2002.02891.x. [DOI] [PubMed] [Google Scholar]
- 23*.Van Duyne GD, Rutherford K. Large serine recombinase domain structure and attachment site binding. Crit Rev Biochem Mol Biol. 2013;48:476–491. doi: 10.3109/10409238.2013.831807. A review of the biochemical and DNA-binding properties of the large serine recombinases, discussed in the context of proposed structural models for the Int-attP and Int-attB complexes. [DOI] [PubMed] [Google Scholar]
- 24.Thorpe HM, Wilson SE, Smith MC. Control of directionality in the site-specific recombination system of the Streptomyces phage phiC31. Mol Microbiol. 2000;38:232–41. doi: 10.1046/j.1365-2958.2000.02142.x. [DOI] [PubMed] [Google Scholar]
- 25.Rowley PA, Smith MCM. Role of the N-terminal domain of phiC31 integrase in attB-attP synapsis. J Bacteriol. 2008;190:6918–21. doi: 10.1128/JB.00612-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis JA, Hatfull GF. Control of directionality in integrase-mediated recombination: examination of recombination directionality factors (RDFs) including Xis and Cox proteins. Nucleic Acids Res. 2001;29:2205–16. doi: 10.1093/nar/29.11.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bibb LA, Hancox MI, Hatfull GF. Integration and excision by the large serine recombinase phiRv1 integrase. Mol Microbiol. 2005;55:1896–910. doi: 10.1111/j.1365-2958.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- 28*.Ghosh P, Wasil LR, Hatfull GF. Control of phage Bxb1 excision by a novel recombination directionality factor. PLoS Biol. 2006;4:e186. doi: 10.1371/journal.pbio.0040186. Demonstration of the contrasting properties of the Bxb1 RDF compared to the RDFs used by the λ-like integrases from the tyrosine recombinase family. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Khaleel T, Younger E, McEwan AR, Varghese AS, Smith MCM. A phage protein that binds φC31 integrase to switch its directionality. Mol Microbiol. 2011;80:1450–63. doi: 10.1111/j.1365-2958.2011.07696.x. Characterization of the φC31 integrase RDF, demonstrating similar biochemical properties compared to the Bxb1 RDF despite the lack of any recognizable sequence similarity. [DOI] [PubMed] [Google Scholar]
- 30.Breüner A, Brøndsted L, Hammer K. Novel organization of genes involved in prophage excision identified in the temperate lactococcal bacteriophage TP901-1. J Bacteriol. 1999;181:7291–7297. doi: 10.1128/jb.181.23.7291-7297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Zhu B, Dai R, Zhao G, Ding X. Control of Directionality in Streptomyces Phage φBT1 Integrase-Mediated Site-Specific Recombination. PLoS ONE. 2013;8:e80434. doi: 10.1371/journal.pone.0080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bushman W, Yin S, Thio LL, Landy A. Determinants of directionality in lambda site-specific recombination. Cell. 1984;39:699–706. doi: 10.1016/0092-8674(84)90477-x. [DOI] [PubMed] [Google Scholar]
- 33.Yin S, Bushman W, Landy A. Interaction of the lambda site-specific recombination protein Xis with attachment site DNA. Proc Natl Acad Sci U S. 1985;82:1040–4. doi: 10.1073/pnas.82.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh P, Bibb LA, Hatfull GF. Two-step site selection for serine-integrase-mediated excision: DNA-directed integrase conformation and central dinucleotide proofreading. Proc Natl Acad Sci U S. 2008;105:3238–43. doi: 10.1073/pnas.0711649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olorunniji FJ, Buck DE, Colloms SD, McEwan AR, Smith MCM, Stark WM, Rosser SJ. Gated rotation mechanism of site-specific recombination by φC31 integrase. Proc Natl Acad Sci U S. 2012;109:19661–6. doi: 10.1073/pnas.1210964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai H, Sun M, Ghosh P, Hatfull GF, Grindley NDF, Marko JF. Single-molecule analysis reveals the molecular bearing mechanism of DNA strand exchange by a serine recombinase. Proc Natl Acad Sci U S. 2011;108:7419–24. doi: 10.1073/pnas.1018436108. [DOI] [PMC free article] [PubMed] [Google Scholar]