Figure 6. Proposed models of paxillin133–290 binding to the FAT domain of FAK and the FAT domain of Pyk2.
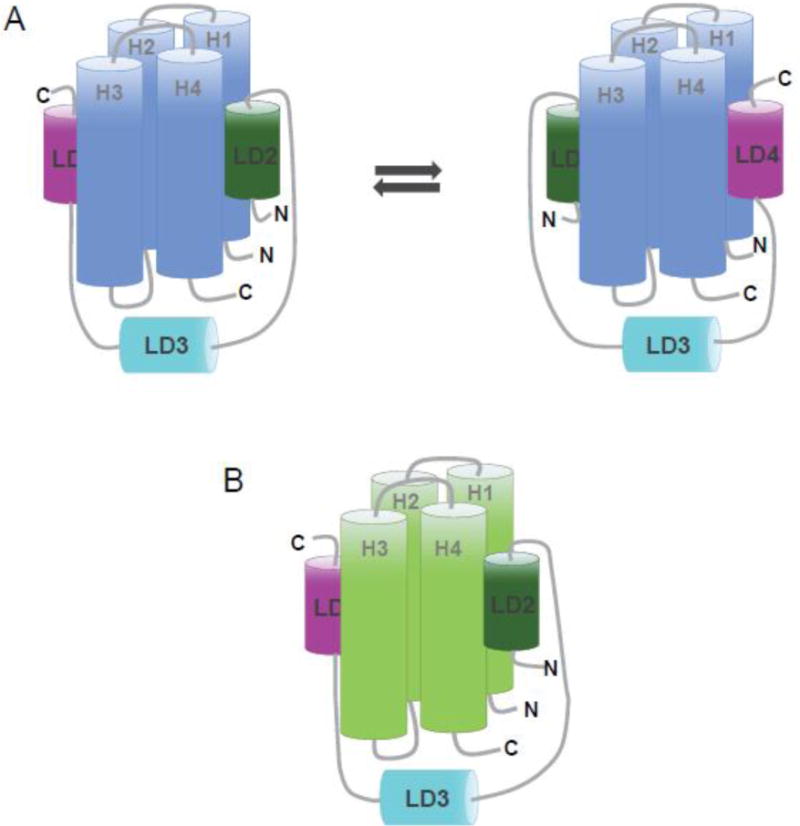
(A) In Pyk2-FAT, the LD2 and LD4 motifs of paxillin133–290 undergo a conformational switching mechanism in which two discrete conformations of paxillin compete for binding; one with LD2 occupying H1/H4 and LD4 occupying H2/H3, and the other conformation with LD4 at H1/H4 and LD2 at H2/H3. (B) In FAT, the LD2 and LD4 motifs of paxillin133–290 binds to opposite faces of the four-helix bundle (H2/H3 and H1/H4) and are oriented in the same direction.
