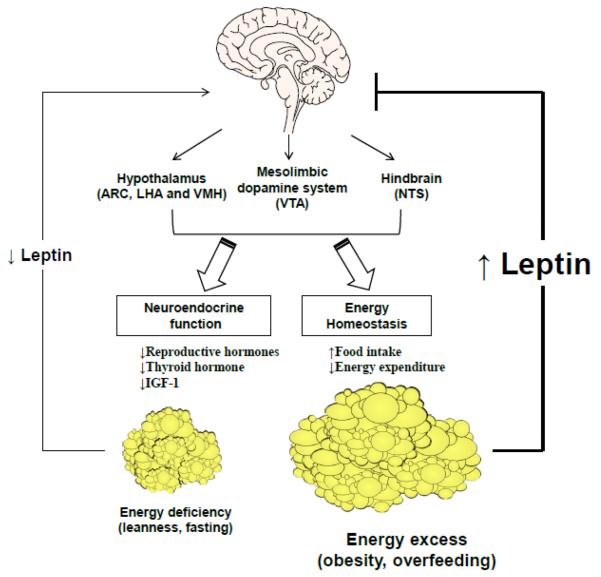
Figure 1. The effects of leptin in states of energy excess and energy deficiency.
In states of energy deficiency such as fasting, circulating leptin levels decrease. As a result, food intake increases due to increased expression of orexigenic neuropeptides and decreased expression of anorexigenic neuropeptides. In addition, the decline of leptin modulates mesolimbic dopamine system and hindbrain circuits to increase food intake, and also has effects on neuroendocrine function and sympathetic nervous system, to decrease energy expenditure. In states of energy excess such as obesity and overfeeding, leptin levels increase; however, leptin's effects in the CNS are blunted due to leptin resistance. Recombinant leptin administration results in improvement in neuroendocrine and metabolic abnormalities in leptin-deficient states such as congenital leptin deficiency, hypothalamic amenorrhea, and congenital or acquired lipoatrophy. On the other hand, in states of leptin excess such as common forms of obesity and type 2 diabetes, recombinant leptin confers minimal benefits, indicating leptin resistance. The latter may be overcome with co-administration of amylin, leptin sensitizers or exercise. Leptin enhances weight loss maintenance in obesity by suppressing food intake and increasing energy expenditure. Abbreviations: ARC, arcuate nucleus; CNS, central nervous system; IGF, insulin-like growth factor; LHA, lateral hypothalamic area; NTS, nucleus of the solitary tract; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.