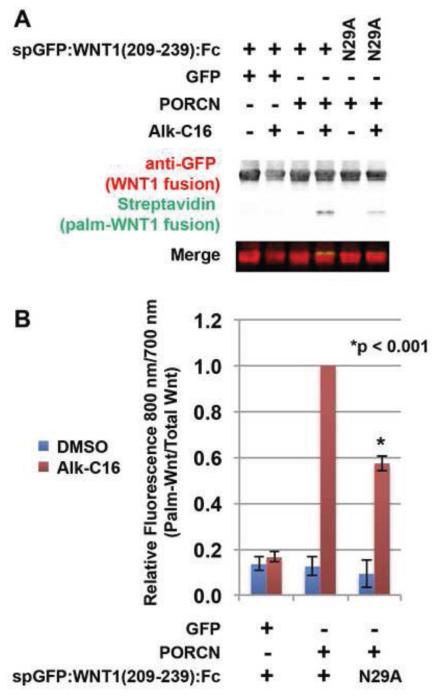
Figure 3. Glycosylation promotes, but is not absolutely required for Wnt1 palmitoylation.
HEK293T cells were co-transfected with Fc fusion constructs encoding wild-type spGFP:WNT1 (209-239) or mutant spGFP:WNT1 (209-239) bearing a N29A mutation, along with GFP (control) or PORCN. The N29A mutation in the Fc fusion protein generates a non-glycosylated variant [19]. Cells were metabolically labeled with DMSO carrier (− Alk-C16) or Alkyne-palmitate (+ Alk-C16) for 24hrs, lysed, and precipitated with protein A/G beads [49]. The beads were then subjected to click chemistry with biotin-azide and then separated by SDS-PAGE and analysis by Western Blot [48]. Western Blots were probed with an anti-GFP antibody followed by Alex680-conjugated secondary (red) and IRDye800-conjugated streptavidin (green). The blot was scanned using a Licor Odyssey CLX scanner. (A) In the presence of PORCN, the glycosylation deficient variant N29A is palmitoylated, albeit at reduced levels. (B) Quantification of relative amounts of palmitate labeled-spGFP:Wnt1:Fc and total spGFP:Wnt1:Fc allows us to conclude that while glycosylation does promote palmitoylation, it is not an absolute prerequisite. Data shown are the average of two (N29A) or four (wild-type) independent replicates. Error bars represent +/− standard error. A Student’s t-test was used to assess statistical significance.