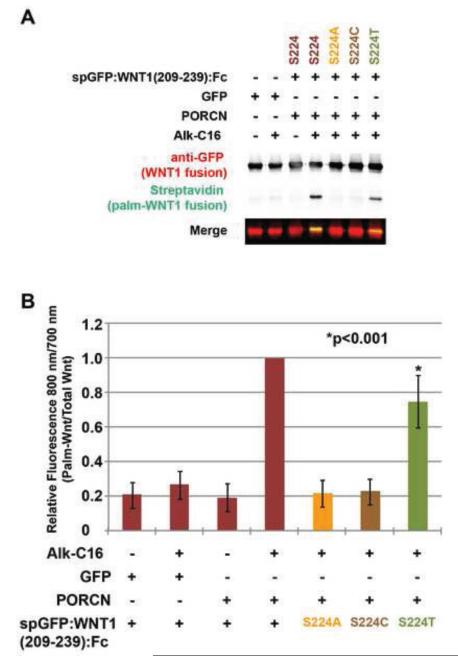
Figure 4. PORCN promotes the palmitoylation of serine, threonine, but not cysteine residues.
HEK293T cells were co-transfected with constructs encoding wild-type and variant spGFP:WNT1(209-239) fusion proteins. The variants possessed S224A, S224C, or S224T substitutions. Cells were metabolically labeled with DMSO carrier (− Alk-C16) or Alkyne-palmitate (+ Alk-C16) for 24hrs. After lysis, Fc containing proteins were precipitated with protein A/G beads. Proteins adhered to the beads were then subjected to bio-orthogonal click chemistry with biotin-azide and then separated by SDS-PAGE and analysis by Western Blot. Western blots were probed with anti-GFP followed by Alex680-conjugated secondary antibody (red) and IRDye800-conjugated streptavidin (green). Blots were scanned using a Licor Odyssey CLX scanner. (A) Overexpression of PORCN in the presence of Alk-C16 promotes the incorporation of Alkyne-palmitate into the wild-type and S224T versions of spGFP:WNT1(209-239). The S224A and S224C variants do not incorporate Alkyne palmitate. (B) Quantitation of relative amounts of incorporated palmitate (800 nm) and GFP (700 nm) shows that PORCN can indeed promote the lipid modification of threonine, but to a lesser degree than for the wild-type protein. Data shown are the average of three independent replicates. Error bars represent +/− standard error. A Student’s t-test was used to assess statistical significance.