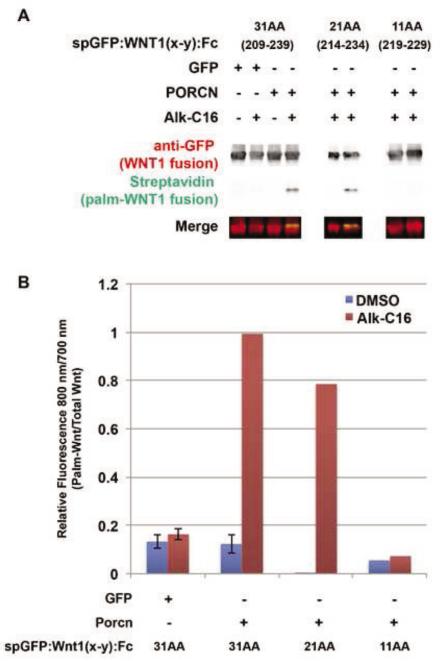
Figure 9. The minimal length of the WNT1 substrate is 21 residues.
HEK293T cells were co-transfected spGFP:WNT1(x-y):Fc fusions proteins with either 31 (209-239), 21 (214-234), or 11 (219-229) amino acids flanking S224, along with GFP (control) or PORCN. Cells were metabolically labeled with DMSO carrier (− Alk-C16) or Alkyne-palmitate (+ Alk-C16) for 24hrs. After lysis, Fc containing proteins were precipitated with protein A/G beads. Proteins bound to the beads were subjected to bio-orthogonal click chemistry with biotin-azide, separated by SDS-PAGE and analyzed by Western Blot. Western Blots were probed with anti-GFP followed by Alexa680-conjugated secondary antibody (red) and IRDye800-conjugated streptavidin (green). The blot was scanned using a Licor Odyssey CLX scanner. (A) Only the 31 and 21 residue Wnt1 fragments are able to incorporate Alkyne palmitate. (B) Quantification of the blots (800 nm/700 nm) shows that the 21 residue fragment incorporates roughly 80% of the palmitate as does the 31 residue fragment. The 11 amino acid peptide does not incorporate any detectable palmitate. Data shown for spGFP:WNT1 (209-239) are the average of four independent replicates. Although data shown for spGFP:WNT1(214-234):Fc and spGFP:WNT1(219-229):Fc are from a single replicate, it should be noted that we also measured the incorporation of 3H-palmitate into spGFP:WNT1(209-239), (214-234), and (219-229) fusion proteins (data not shown). Similar results were obtained using both assays (incorporation of Alk-C16 or 3H-palmitate). Error bars represent +/− standard error. A Student’s t-test was used to assess statistical significance.