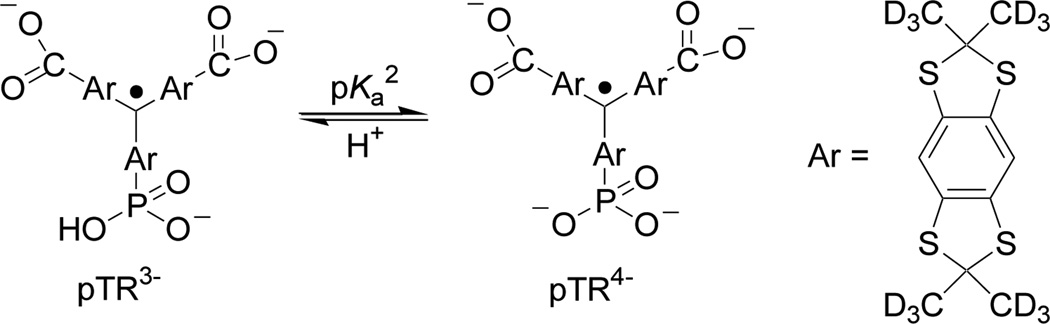
Scheme 1.
Chemical structure of monophosphonated trityl radical, pTR, and the scheme of acid-base equilibrium between the radical forms with protonated (pTR3−) and unprotonated (pTR4−) phosphono group (pKa2=6.9). Note the values of pKa1≈1.3 for the first dissociation of phosphono group and pKa≈2.6 for the dissociation of carboxyl groups [16].