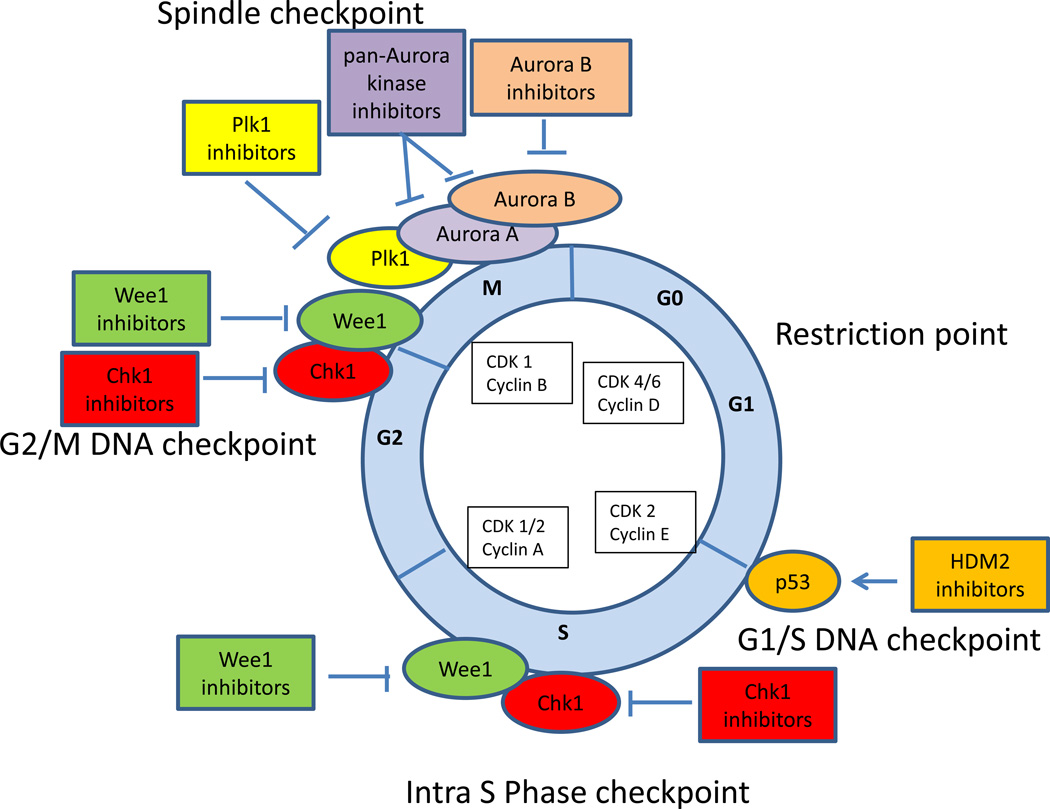
Figure 1.
Cell cycle checkpoint inhibitors in AML. Cell cycle is controlled by successive activation of cyclin-cyclin dependent kinases (CDKs). Cell cycle checkpoints serve to stop progression of the cell cycle in response to DNA damage, to allow time for DNA repair and to preserve genomic integrity. The p53-dependent G1/S checkpoint blocks initiation of DNA replication. p53 is frequently altered (mutations or losses) in patients with complex karyotype AML. Inhibitors of HDM2 (RG7112, RO5503781 and MK-8242) are in clinical studies in AML alone or in combination with cytarabine and have a potential to be effective in leukemia cells that retain wild-type p53. Tumors that are defective in p53 function rely on an intact S phase and G2 checkpoint. Depending on the type of genotoxic stress, either ataxia-telangectasia mutated (ATM) protein kinase or ataxia-telangiectasia-related (ATR) protein kinase are activated leading to activation of Chk1 and Wee1 kinases that inactivate Cdk1 resulting in intra S phase and G2/M arrest, allowing time for DNA repair. Inhibition of Chk1 (MK8776) and Wee1 (MK1775) sensitizes leukemia cells to cytarabine cytotoxicity in preclinical studies and the combination of Chk1 inhibitor and cytarabine is in a phase II testing in AML. Synergistic activity has been described for Chk1 and Wee1 inhibition in leukemia cells. PLK1 and Aurora kinases are critical for centrosome maturation and proper formation of the mitotic spindle, and also play a role in chromosome segregation and cytokinesis. Plk1 inhibitor (volasertib) and Aurora B kinase inhibitor (AZD1152) have shown promising clinical activity in AML patients when given with low dose cytarabine. In addition, several pan CDK inhibitors (flavopiridol, dinaciclib) have been explored as a therapeutic strategy in AML. Flavopiridol in combination with cytarabine and mitoxantrone (FLAM) is in a phase III testing for newly diagnosed AML.