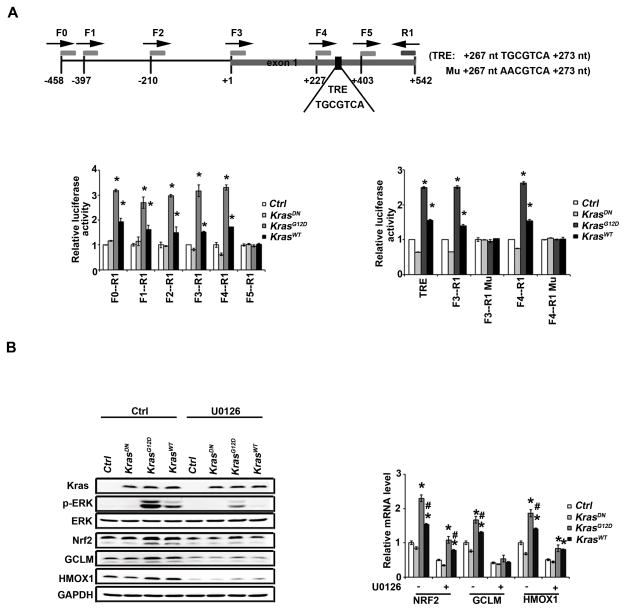
Figure 3.
KRAS transcriptionally activates NRF2 through the TRE. (A) Identification of a TRE (267 nt TGCGTCA 273 nt) in the promoter of NRF2. The different human NRF2 promoter constructs were cloned upstream of a luciferase reporter gene (the sites where primer pairs bind are illustrated). These constructs were co-transfected into HEK293 cells alone with a control, KRASDN, KRASG12D or KRASWT expression vector for 48h. Dual luciferase activities were measured. The experiment was repeated three times, each with triplicate samples. Data are expressed as mean ± SEM (*p< 0.05 Ctrl group vs. KRAS) (left panel). TRE refers to a construct where the TRE sequence TGCGTAC flanked by 15 nt on both sides was inserted into the cloning site of the luciferase reporter gene vector (left panel). F3-R1 Mu or F4-R1 Mu is a construct where the TRE sequence (TGCGTCA) in the F3-R1 or F4-R1 construct was mutated to AACGTCA. Dual luciferase activities with these constructs were measured as described (right panel). (B) KRAS upregulated NRF2 and its target genes through activation of ERK. HEK293 cells were either transfected with empty vector, KRASDN, KRASG12D or KRASWT for 24 h. Cells were treated with 10 μM U0126 for 4 h after overnight starvation. mRNAs were extracted and the relative mRNA level of NRF2, GCLM, HMOX1 was then determined by quantitative real-time RT-PCR. The experiment was repeated three times, each with triplicate samples. The data are expressed as mean ± SEM (*p< 0.05 Ctrl group vs. KRAS) (upper panel). Cell lysates from another set of the same experiment were subjected to immunoblot analysis (lower panel).