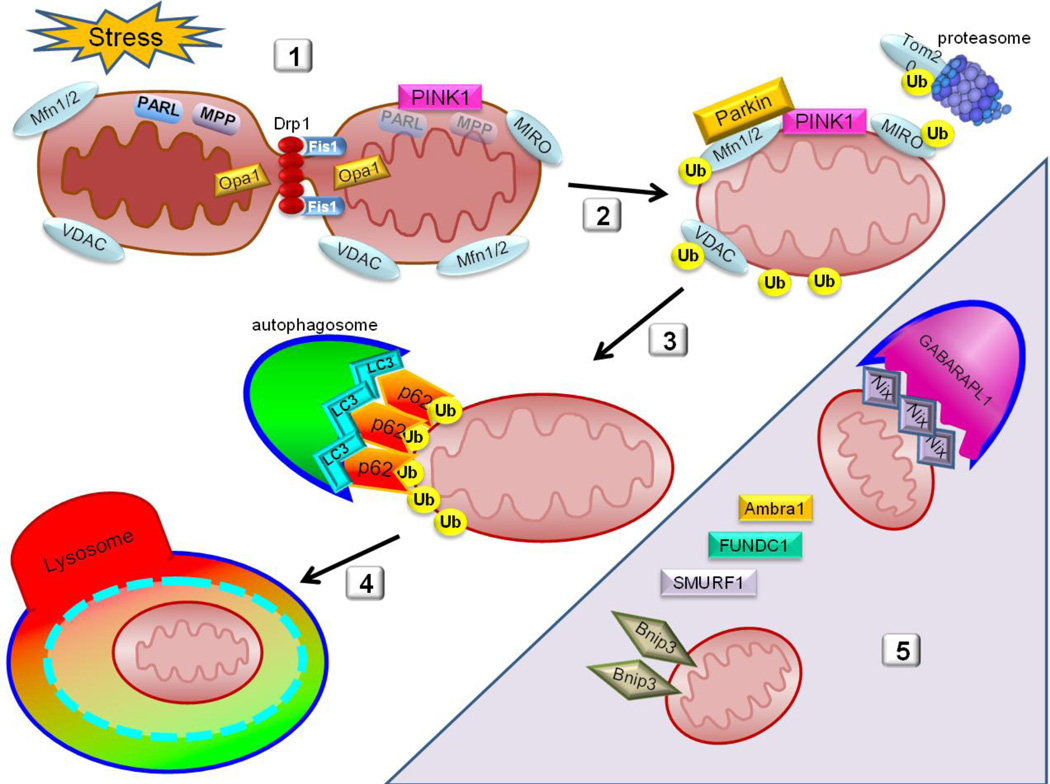
Cellular stress, such as ischemia/reperfusion, triggers fragmentation of the mitochondria mediated by Drp1, segregating low-membrane potential mitochondria from the rest of the network. Ischemia/reperfusion injury also leads to the collapse of mitochondrial membrane potential which deactivates PARL and MPP, allowing for PINK1 stabilization on the OMM.
Parkin is recruited to the OMM where it binds Mfn2 and ubiquitinates multiple OMM proteins, marking them for proteasomal degradation and targeted recognition of the ubiquitin-decorated mitochondrion.
Autophagy adapter proteins such as p62 are then recruited to the mitochondria which in turn bind the ubiquitinated mitochondrion to the phagophore through interaction with LC3 or homologs.
Once the autophagosome has fully engulfed the mitochondrion, it fuses with a lysosome to form the autophagolysosome where final degradation of bulk contents is completed.
Shaded area indicates atypical players that participate in recognition and targeting of mitochondria for autophagic clearance. These include Nix and Bnip3 which bind LC3 or homologs including GABARAPL1.