Abstract
Objectives:
To find preoperative predictive signs for better surgical planning of the facial nerve in parotid tumors.
Methods:
Prospective study in patients with primary parotid malignancies. Patients with primary parotid malignant tumor were investigated for preoperative clinical signs in correlation with histological findings and surgical management of the facial nerve.
Outcomes:
The study included 47 patients. Several clinical findings as facial pain, paresthesia, and rapid growth of tumor might suggest the risk of malignancy. Paresis/palsy of the facial nerve was correlates with direct neural involvement.
Conclusion:
There are several predictive clinical signs that might suggest malignancy of a parotid tumor.
Keywords: pain, paresthesia, growth, palsy, facial, parotid malignancy
INTRODUCTION
In light of the dramatic consequences of facial nerve resection in terms of function and quality of life, preoperative knowledge of facial nerve involvement can help for better planning including microsurgical team and patient preparation (1). Moreover, early detection of malignancy allows improving surgical planning and information given to patients. Histological pre-diagnosis can then be obtained by cytological analysis of fine needle aspiration (FNAC) (2) and frozen sections during the parotidectomies (3). Preoperative facial nerve palsy is a definite indication for its involvement by the tumor, but when the facial nerve function is intact there are no accurate preoperative known criteria for prediction of its involvement (1).
In this prospective study the authors aimed to find preoperative predictive factors for better surgical planning of the facial nerve in correlation with FNAC and/or frozen sections, and finally, with definitive histopathological exam. ❑
MATERIAL AND METHODS
The institutional review board of "Iuliu Hatieganu" University of Medicine and Pharmacy, Cluj-Napoca, Romania, approved the present study. The study was conducted between January 2007 and January 2012 at the Department of ENT of the Emergency County Hospital, Cluj-Napoca, Romania. The surgeon performed superficial or total parotidectomies with or without facial nerve sacrifice by following the same protocol. A senior pathologist from the Department of Pathology from the Emergency County Hospital, Cluj-Napoca, Romania, performed the histologic examination.
The patients involved in the study first signed an informed consent for participation. The patients were included in the study by observing the following inclusion criteria: (1) primary parotid tumor and (2) primary surgical resection.
However, we excluded patients with any prior parotid surgery or non-primary parotid tumor, prior radiotherapy/chemotherapy, patients who had synchronous or metachronous head or neck malignancies, and patients who were subject to follow-ups for less than four years from the present study. The patients who refused surgery were not included in the study.
The following data were observed during the study: patient age and gender, facial pain at presentation (daily intake of pain medication); paresthesia; rapid growth of tumor (double size of the tumor in the last month); paresis or facial nerve palsy; fine needle aspiration cytology (FNAC) preoperatively; frozen section; type of surgical procedure; findings of facial nerve involvement at surgery; histological findings; lymph node involvement; postoperative radiation therapy; postoperative facial palsy; and recurrence of tumor. During this period, FNAC and frozen sections were performed by the same pathologist well trained to these techniques. FNAC was performed in patients who have given their consent for this labor, some of them refusing FNAC for various reasons: they were frightened, lived far from the hospital, didn't understand its goal, and refused to know the diagnosis before the operation. To all patients included in the study were performed frozen sections. After comparison of the FNAC and frozen sections findings with the final histological results, we evaluated both the precision of the tumor histological diagnosis and the capacity to detect malignancy related to symptoms and clinical signs.
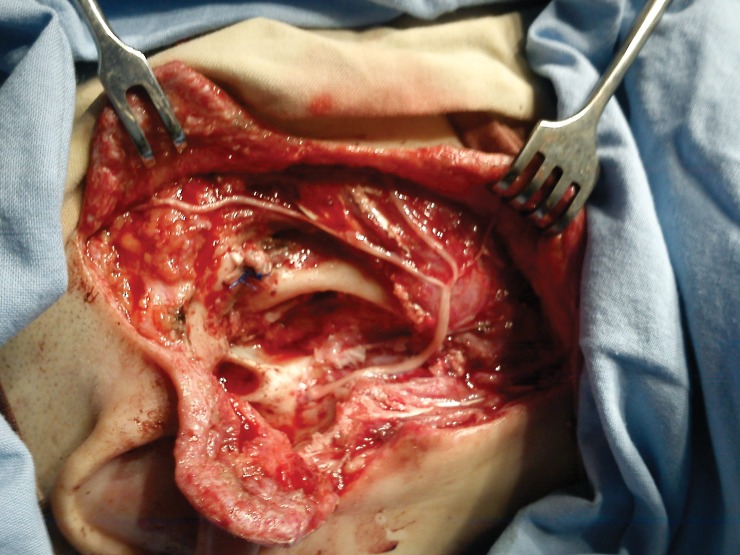
All parotidectomies were performed by the head and neck surgeon and the facial nerve was identified by standards landmarks (tragal pointer, posterior belly of the digastrics muscle and the tympanomastoid suture line), without facial nerve monitoring. The facial nerve trunk or branches that were encased within the tumor were included in the resection. For facial reanimation a cable graft was harvested from the sural nerves, and microscopic epineural repair was done with 9.0 nylon sutures (Figure 1).
Figure 1. Shows the facial rehabilitation with sural cable graft.
The statistical analysis was performed using Statistica 8.0 software (StatSoft, Inc., Tulsa, OK 74104) with a significant level less than 0.05. Pearson Chi-square test was used to compare variables between groups, and odds ratio, risk ratio, Fisher exact test, and Mann-Whitney U tests were used for continuous variables. ❑
OUTCOMES
Forty-seven patients were included in the study, all of them signing an informed consent for participation. The study included nineteen women with a mean age 54.68 years (95% CI: 49.50-59.87) and twenty eight men with a mean age of 59.36 years (95% CI: 55.60-63.12), with no difference between the age of appearance of parotid gland pathology (T-tests, p=0.127703).
In Table 1 we can see the parotid gland pathology included in this study, after final histological data. FNAC was performed at twenty three patients; in nineteen cases we had true positive results in comparison with final histological diagnosis. The frozen sections offered about 100% of true positive results compared with final histology, with high statistical significance (Pearson Chi-Square test: kappa = 0.913, p<0.0001).
Table 1.
Histological types of parotid tumor
| Type | Frequency | % |
|---|---|---|
| Pleomorphic adenoma | 11 | 23.4 |
| Whartin tumor | 5 | 10.6 |
| Oncocytoma | 2 | 4.3 |
| Inverted ductal papilloma | 2 | 4.3 |
| Mucoepidermoid carcinoma | 6 | 12.8 |
| Acinic cell carcinoma | 4 | 8.5 |
| Adenoid cystic carcinoma | 2 | 4.3 |
| Polymorphic adenocarcinoma | 2 | 4.3 |
| Squamous cell carcinoma ex-pleomorphic adenoma | 8 | 17.0 |
| Salivary duct carcinoma | 1 | 2.1 |
| Papillary cystadenocarcinoma | 3 | 6.4 |
| Sialadenosis | 1 | 2.1 |
| Total= 47 | 100.0 |
Twenty patients (42.6%) underwent superficial parotidectomy with facial nerve dissection, all for benign lesions, while twenty seven (57.4%) underwent total parotidectomy, in seven cases with facial nerve sacrifice and facial reanimation. Fifteen patients experienced postoperative nerve dysfunction (paresis or paralysis). In fourteen cases were performed selective neck dissections (29.8%), and eleven patients underwent comprehensive neck dissections (23.4%) respective twenty two patients had no neck dissection (46.8%); the decision to perform neck dissection was taken depending on the outcome of the frozen sections (selective or comprehensive neck dissection for medium or high grade of malignancy). All patients with total parotidectomy and neck dissection performed postoperative radiotherapy in agreement with the decision of the head and neck oncology multidisciplinary committee.
The average age of those patients with benign pathology was statistically significant lower than the average age of the patients with low grade malignant disease (T-tests: p = 0.023581). Instead, the average age of patients with benign pathology does not differ significantly from the average age of persons with high grade malignant disease (T-tests: p = 0.729205). The average age of those with low grade malignant disease is significantly higher compared with the average age of patients with high grade malignancy (T-tests: p = 0.007568).
In this study we followed a series of clinical symptoms and signs listed in Table 2, in order to study their predictive value for malignancy. Because not for all patients included in the study was performed FNAC, clinical correlation with symptoms and signs were made with the frozen sections.
Table 2.
Clinical symptoms and signs
| present | absent | present (%) vs absent (%) (p value) |
|
|---|---|---|---|
| Facial pain | 3 | 44 | 0.00 vs 0.14 (0.1735) |
| Paresthesia | 14 | 33 | 0.62 vs 0.64 (0.9152) |
| Rapid growth of the tumor (double size in the last month) | 16 | 31 | 0.38 vs 0.71 (0.0973) |
| Paresis/palsy of the facial nerve | 4 | 43 | 0.08 vs 0.21 (0.3502) |
Table 3.
Predictive value of clinical symptoms and signs
|
Odds Ratio (OR) |
Risk Ratio (RR) |
Fisher exact test (p value) |
|
|---|---|---|---|
| Facial pain | 0.9740 | 0.4502 | 0.4988 |
| Paresthesia | 1.8750 | 1.3500 | 0.5000 |
| Rapid growth | 1.5556 | 1.3333 | 0.56643 |
| Facial paresis/palsy | 9.3333 | 2.0417 | 0.01836 |
Regarding the predictive value of sacrificing the facial nerve in relation to appearance of recurrence, statistical tests showed no statistically significant difference in recurrence rate in patients with or without facial nerve sacrifice (OR = 4.000; RR = 1.7500; Fisher exact test: p = 0.1392).
There was no statistically significant difference between the 4 years survival of the patients with or without facial nerve sacrifice (Mann-Whitney U test: p = 0.219) or between the 4 years survival of the patients with or without macroscopically invasion of the facial nerve (Mann-Whitney U test: p = 0.325). ❑
DISCUSSION
The malignant lesions of the parotid gland are histologically and biologically variable, mucoepidermoid, adenoid cystic, and acinic cell carcinomas are the most prevalent (1). The gold standard treatment for the parotid gland tumor has been the parotidectomy, traditionally radical but more conservative since there is the recent advances in understanding of tumor biology and the benefits of radiotherapy (1, 4, 5). Today there is an agreement of the most head and neck surgeons that the facial nerve can be spared providing the nerve is not involved; the nerve should be sacrifice if the tumor is adherent to or surround the nerve (1).
In the present study, all patients with malignant tumor at the frozen sections underwent total parotidectomy, seven of them with facial nerve sacrifice. The facial nerve sacrificing decision was taken considering the following reasons: adenoid cystic carcinoma (2 cases) recognized for perineural extension, 3 cases of high-grade mucoepidermoid carcinoma and 2 cases of squamous cell carcinoma ex-pleomorphic adenoma, in all those 5 cases the nerve being embedded in the tumor without any possibility of dissection.
In 2010, Preis and colab. (2) did not find any difference in age, gender, pain at presentation and presence of positive lymph nodes between patients with or without facial nerve involvement, studying a lot of 267 parotidectomies with predominance of acinic cell, ex-pleomorphic, and salivary duct carcinomas. They concluded that early prediction of a high risk of facial nerve involvement by malignant parotid tumors is important for treatment planning and patient counseling. Only facial palsy at presentation is the only predictor of invasion of the facial nerve by tumor cells.
There are a number of studies in English literature that identified some factors with predictive value for facial nerve management: advanced age, deep lobe involvement, large size of the tumor, a low response to preoperative electroneuronography, a high negative predictive value for cross-sectional imaging with computed tomography and magnetic resonance imaging (6-11). Most studies in recent years focused on the predictive value of FNAC and frozen sections in surgical management of the facial nerve, considering that only preoperative predictive clinical signs at presentation is facial paralysis (3,12-15).
Our study was focused on the primary parotid malignancies, because for non-primary parotid malignancies the clinical presentation, surgical management and outcomes are different, with better prognosis of facial nerve function (16). In addition, we focused our study on the clinical symptoms and signs at presentation, which can guide diagnosis and therapy planning in front of a parotid gland mass. The invasion of the deep parotid lobe or tumors which extend into the parapharyngeal space, recurrent tumors, skin involvement or extension into the bone, locally extensive lesions, and the presence of the pathologic neck nodes are gross clinical or imagistic signs associated with malignancy. The sacrifice of the facial nerve erodes the quality of life, so preoperative patient counseling and rehabilitation of facial nerve become mandatory for head and neck surgeons.
The results of our study show that high-grade parotid malignancies occur at a mean age of 54.28 years, older age being the attribute of the benign or low-grade malignancies. The facial pain and paresthesia, rapid growth of tumor, and facial paresis/palsy are clinical symptoms and signs that have not a high frequency but may suggest malignancy, especially paresis or paralysis of the facial nerve which means direct neural involvement. Also, our study confirms that the facial nerve sacrifice doesn't bring benefits in the survival of the parotid tumors' patients. ❑
CONCLUSIONS
Middle-age, facial pain and paresthesia, and rapid growth of the parotid tumor might suggest the malignancy with consequences in patient's counseling and facial nerve surgical management.
The paresis or paralysis of the facial nerve at presentation means direct neural involvement.
The sacrificing of the facial nerve doesn't improve local control and survival of patients with parotid malignancies.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Preis M, Soudry E, Bachar G, et al. Predicting facial nerve invasion by parotid gland carcinoma and outcome of facial reanimation. Eur Arch Otorhinolaryngol. 2010;276:107–111. doi: 10.1007/s00405-009-0968-x. [DOI] [PubMed] [Google Scholar]
- 2.Deneuve S, Quesnel S, Depondt J, et al. Management of parotid gland surgery in a university teaching hospital. Eur Arch Otorhinolaryngol. 2010;276:601–605. doi: 10.1007/s00405-009-1088-3. [DOI] [PubMed] [Google Scholar]
- 3.Zbären P, Guélat D, Loosli H, et al. Parotid tumors: fine-needle aspiration and/or frozen section. Otolaryngol Head Neck Surg. 2008;139:811–815. doi: 10.1016/j.otohns.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Spiro JD, Spiro RH. Cancer of the parotid gland: role of 7th nerve preservation. World J Surg. 2003;27:863–867. doi: 10.1007/s00268-003-7112-7. [DOI] [PubMed] [Google Scholar]
- 5.Spiro RH. Salivary neoplasms: overview of a 35 year experience with 2,807 patients. Head Neck Surg. 1986;8:77–84. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 6.Gaillard C, Perie S, Susini B, et al. Facial nerve dysfunction after parotidectomy: the role of local factors. Laryngoscope. 2005;115:287–291. doi: 10.1097/01.mlg.0000154735.61775.cd. [DOI] [PubMed] [Google Scholar]
- 7.Laccourreye H, Laccourreye O, Cauchois R, et al. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25-year experience with 229 patients. Laryngoscope. 1994;104:1487–1494. doi: 10.1288/00005537-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Marchesi M, BiVoni M, Trinchi S, et al. Facial nerve function after parotidectomy for neoplasms with deep lobe localization. Surg Today. 2006;36:308–311. doi: 10.1007/s00595-005-3146-9. [DOI] [PubMed] [Google Scholar]
- 9.Dulguerov P, Marchal F, Lehmann W. Postparotidectomy facial nerve paralysis: possible etiologic factors and results with routine facial nerve monitoring. Laryngoscope. 1999;109:754–762. doi: 10.1097/00005537-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Bendet E, Talmi YP, Kronenberg J. Preoperative electroneurography (ENoG) in parotid surgery: assessment of facial nerve outcome and involvement by tumor – a preliminary study. Head Neck. 1998;20:124–131. doi: 10.1002/(sici)1097-0347(199803)20:2<124::aid-hed5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Divi V, Fatt MA, Mukherji SK, et al. Use of cross-sectional imaging in predicting facial nerve sacriWce during surgery for parotid neoplasms. ORL J Otorhinolaryngol Relat Spec. 2004;66:262–266. doi: 10.1159/000081123. [DOI] [PubMed] [Google Scholar]
- 12.Al-Khafaji BM, Nestok BR, Katz RL. Fine-needle aspiration of 154 parotid masses with histologic correlation: ten-year experience at the University of Texas M. D. Anderson Cancer Center. Cancer. 1998;84:153–159. [PubMed] [Google Scholar]
- 13.Salgarelli AC, Capparé P, Bellini P, et al. Usefulness of fine-needle aspiration in parotid diagnostics. Oral Maxillofac Surg. 2009;13:185–190. doi: 10.1007/s10006-009-0182-4. [DOI] [PubMed] [Google Scholar]
- 14.Harish K. Management of primary malignant epithelial parotid tumors. Surg Oncol. 2004;13:7–16. doi: 10.1016/j.suronc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Jafari A, Royer B, Lefevre M, et al. Value of the cytological diagnosis in the treatment of parotid tumors. Otolaryngol Head & Neck Surg. 2009;140:381–385. doi: 10.1016/j.otohns.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Périé S, St Guily JL. Primary and non-primary parotid malignancies: comparison of treatment modalities and outcomes. Eur Arch Otorhinolaryngol. 2007;264:1231–1237. doi: 10.1007/s00405-007-0351-8. [DOI] [PubMed] [Google Scholar]