Abstract
Background
To determine optimal methods of Respiratory Inductance Plethysmography (RIP) flow calibration for application to pediatric post-extubation upper airway obstruction.
Methods
We measured RIP, spirometry, and esophageal manometry in spontaneously breathing, intubated Rhesus monkeys with increasing inspiratory resistance. RIP calibration was based on: ΔµVao ≈ M[ΔµVRC + K(ΔµVAB)] where K establishes the relationship between the uncalibrated rib cage (ΔµVRC) and abdominal (ΔµVAB) RIP signals. We calculated K during: (1) isovolume maneuvers during a negative inspiratory force (NIF) (2) Quantitative Diagnostic Calibration (QDC) during (a) tidal breathing, (b) continuous positive airway pressure (CPAP), and (c) increasing degrees of UAO. We compared the calibrated RIP flow waveform to spirometry quantitatively and qualitatively.
Results
Isovolume calibrated RIP flow tracings were more accurate (against spirometry) both quantitatively and qualitatively than those from QDC (p<0.0001), with bigger differences as UAO worsened. Isovolume calibration yielded nearly identical clinical interpretation of inspiratory flow limitation as spirometry.
Conclusions
In an animal model of pediatric UAO, Isovolume calibrated RIP flow tracings are accurate against spirometry. QDC during tidal breathing yields poor RIP flow calibration, particularly as UAO worsens. Routine use of a NIF maneuver before extubation affords the opportunity to use RIP to study post extubation UAO in children.
Introduction
Upper airway obstruction (UAO) is common to a variety of pediatric diseases (1, 2), and is frequent after endotracheal extubation. Definitive data on risk factors and prevention of pediatric post-extubation UAO are lacking. This may relate to poor clinical acumen in distinguishing UAO from residual pulmonary or neuromuscular disease (3). More objective measures of post-extubation UAO severity in infants and children may help identify risk factors and elucidate optimal treatment or prevention strategies (1, 3–5).
Inspiratory flow limitation is relatively specific to extrathoracic UAO, characterized by disproportionately large inspiratory effort relative to flow. The most widely accepted method to measure flow is spirometry, which for non-cooperative spontaneously breathing children requires a tight fitting mask over the nose and mouth, which may require sedation and change flow dynamics (1, 4).
Respiratory inductance plethysmography (RIP) is a less invasive alternative to spirometry. With RIP, variations in the self-inductance of a coil (wires around the ribcage (RC) and abdomen (ABD)) are measured as a result of changes in the cross sectional area of the RC and ABD (6–8). However, RIP signals must be calibrated for accurate values of flow. Some RIP calibration techniques require specific respiratory maneuvers to generate isovolume conditions (a period of effort with no air flow), while others can be applied to tidal breathing (9, 10).
Some have demonstrated in adults that RIP calibration factors generated from isovolume maneuvers are similar to those obtained during 5 minutes of tidal breathing (using Quantitative Diagnostic Calibration (QDC)) (11) and that QDC calibrated RIP signals combined with esophageal manometry can detect flow-limitation for adults with obstructive sleep apnea (5). Others have found conflicting results (12–14). In children, QDC calibration may work with minimal respiratory disease (15, 16). However, given differences in the elastic properties of the chest wall in children compared to adults, it is unclear if the relationship between RC and ABD compartments is stable in children as inspiratory load increases. This may mean that QDC calibration of RIP signals in children is not accurate under increasing inspiratory load. The need for isovolume calibration limits the application of RIP in spontaneously breathing infants. However, it may be possible to perform an isovolume calibration on spontaneously breathing intubated children prior to extubation, affording its use to study post-extubation UAO.
We hypothesized that the combination of calibrated RIP and esophageal manometry will provide an objective measure of the severity of post-extubation UAO. However, several questions regarding RIP calibration limit its immediate application to pediatric post extubation UAO and are the focus of this investigation. 1) Which method of calibration (isovolume versus QDC) is more accurate to detect extrathoracic UAO in children? 2) Can we achieve isovolume conditions during a Negative Inspiratory Force (NIF) procedure, which is routinely done prior to extubation? 3) Do various levels of Continuous Positive Airway Pressure (CPAP) affect QDC calibration, which may preclude QDC calibration prior to extubation? 4) Can QDC calibration of RIP flow occur during ongoing UAO, eliminating the need for measurements prior to extubation?
We sought to test these questions using an established Rhesus monkey model of pediatric UAO (17, 18).
Results
We used 10 Rhesus monkeys, all of which tolerated the experiment, with no complications, were extubated, and returned to their pre-experiment state of health.
Primary Objective: Accuracy of calibration (Isovolume versus QDC) during increasing UAO
We applied calibration factors from the Negative Inspiratory Force (NIF) maneuver at Functional Residual Capacity (FRC) (Isovolume) and Quantitative Diagnostic Calibration (QDC) during Continuous Positive Airway Pressure (CPAP) of 5 cmH2O, to conditions of increasing UAO from fixed calibrated inspiratory resistors. Using quantitative analysis, at every level of resistance, isovolume calibration resulted in a lower mean square error (judged against spirometry), to calibration using QDC on CPAP of 5 cmH2O (p<0.0001) (Figure 1). The same findings held using qualitative methods (p<0.0003). On average, with even mild increase in UAO, QDC yielded clinically inadequate calibration of flow tracings, with 76% of observations (127/168) falling into the terrible or poor category. In contrast, isovolume calibration resulted in good or excellent calibration for all levels of inspiratory resistance with only 2.4% (4/168) of observations being inadequate.
Figure 1. Box plot of inspiratory Mean Squared Error between RIP and Spirometry per resistor by Calibration procedure.

RIP flow pattern compared to gold standard of spirometry using two different RIP calibration techniques (Isovolume maneuver with a NIF (Isocal) versus QDC calibration on CPAP of 5cmH2O (QDCCPAP)) with increasing degrees of UAO. Note the improved accuracy (lower MSE) for RIP calibration using isovolume conditions compared to QDC calibration on CPAP, particularly as UAO increases (all p<0.0001). Bar is median, box is Inter Quartile Range (IQR), whisker is non-outlier range. Circle and unfilled box is Isovolume, Square and grey box is QDC on CPAP of 5cmH2O.
Reproducibility and accuracy of calibration generated from an isovolume maneuver
For all 10 monkeys at both Functional Residual Capacity (FRC or end- expiration) and end inspiration, we achieved an isovolume condition with no flow seen on spirometry, negative deflections in esophageal pressure, and Respiratory Inductance Plethysmography (RIP) tracings of Ribcage (RC) and Abdomen (ABD) which were nearly 180 degrees out of phase (opposite directions of each other) (Figure 2).
Figure 2. Example of NIF to achieve isovolume maneuver (10 breaths).
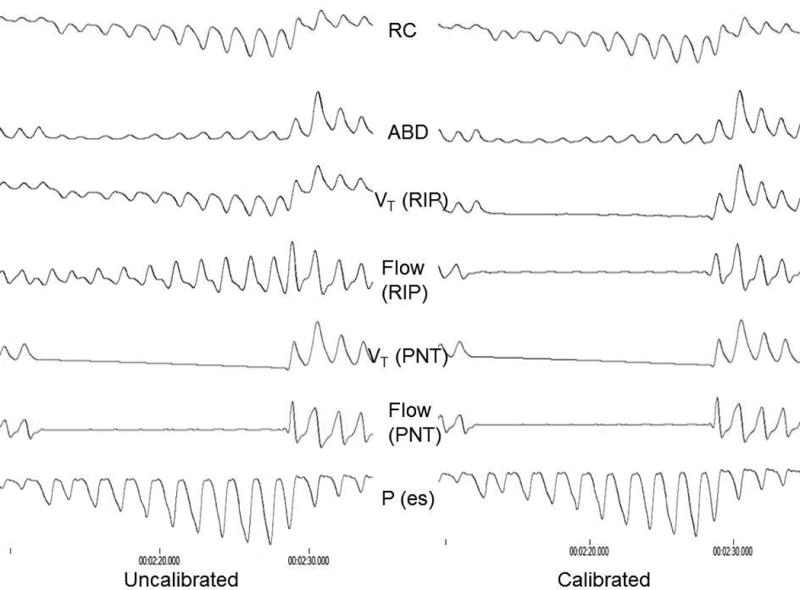
The left panel represents the raw, uncalibrated signals. Notice isovolume conditions are achieved (RC and ABD opposite directions to each other, no flow on spirometry (PNT), and negative deflections in esophageal pressure (Pes)). The right panel represents the same maneuver after RIP calibration using the calibration factor determined from the NIF. Notice how the RIP flow and volume (Vt) tracings more closely represent the zero flow seen on spirometry after calibration.
For the three cycles of obstruction maneuvers at both lung volumes, nine of the 10 monkeys had variation in calibration factors (K) between maneuvers of < 10% (range 0–9.5%, median 4.5 and 5%). One monkey had a 52% difference in K values generated during the three maneuvers at FRC and 41% difference at end inspiration.
Table 1 compares K values at FRC and end inspiration and the accuracy of RIP flow signal calibration (compared to spirometry) using each of the K values during isovolume maneuvers. In general, the K values generated at FRC and end inspiration were similar (p =0.09). During the isovolume maneuver RIP flow (which should be close to zero) was a median of 25% of the flow seen during tidal breathing (IQR 12, 36) at FRC and 30% (IQR 14.4,80) at IC (Figure 3). Qualitative analysis demonstrated at FRC, 7 monkeys had excellent calibration, two good, and 1 adequate. At end inspiration, 7 monkeys had excellent calibration, 1 good, 1 adequate, and 1 bad.
Table 1.
RIP flow calibration factors (K) generated during an isovolume maneuver at FRC and End inspiration, as well as K values generated using QDC calibration under a variety of conditions. Baseline (unobstructed breathing), CPAP continuous positive airway pressure, values in cmH2O. UAO upper airway obstruction, calibrated linear resistor placed on the inspiratory limb of the breathing circuit, values in cmH2O/L/sec.
| Monkey | FRC | End Insp | Baseline | CPAP 5 | CPAP 10 | CPAP 15 | UAO 50 | UAO 200 |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.6 | 2.6 | 0.7 | 0.6 | 0.6 | 0.9 | 0.9 | 1 |
| 2 | 4.5 | 4.5 | 0.8 | 1.2 | 0.9 | 1.1 | 0.9 | 1.8 |
| 3 | 2.7 | 2.7 | 0.7 | 0.7 | 0.5 | 0.7 | 0.5 | 0.8 |
| 4 | 4.7 | 4.3 | 1 | 0.4 | 0.5 | 1 | 1 | 1 |
| 5 | 5 | 4.8 | 0.9 | 0.9 | 0.7 | 1.9 | 0.7 | 1.1 |
| 6 | 1.5 | 1.3 | 0.3 | 0.4 | 0.5 | 0.6 | 0.4 | 0.7 |
| 7 | 2.7 | 2.5 | 1 | 0.6 | 0.7 | 0.9 | 0.9 | 1.8 |
| 8 | 22 | 17 | 1.1 | 1.1 | 0.6 | 0.8 | 0.7 | 4.6 |
| 9 | 7.4 | 6.3 | 0.6 | 1 | 0.7 | 0.8 | 1.1 | 3.6 |
| 10 | 3.3 | 1 | 0.8 | 0.8 | 0.9 | 0.8 | 0.9 | 1.5 |
Figure 3. The percentage of flow (compared to tidal breathing) which would be seen during the NIF maneuver, based on RIP calibration technique.

Zero flow was confirmed with spirometry, so the closer the values are to zero, the more accurate the RIP flow calibration. There was no difference in the percent of RIP flow seen at the two lung volumes (FRC and IC) when applying calibration factors generated from the isovolume maneuver (Iso_FRC and Iso_IC) (p>0.2). There was also no difference in the percent of RIP flow seen at the two lung volumes (FRC and IC) when applying calibration factors generated from the QDC calibration on CPAP (QDC_FRC and QDC_IC) (p>0.2). However, isovolume calibration was superior to QDC, at both lung volumes (p<0.002). Bar is median, box is Inter Quartile Range (IQR), whisker is non-outlier range. Triangles are raw data.
Calibration factors using QDC during unobstructed tidal breathing, CPAP 5, 10, 15 cmH2O
K values generated using QDC on the three different levels of CPAP were not substantially different from each other, or than those generated during baseline conditions with no added CPAP (all p > 0.22, Table 1, Figure 4).
Figure 4. Box Plot of Calibration Factors (K) by condition.

K values generated during Isovolume conditions at FRC and End inspiration, as well as using QDC calibration under various degrees of CPAP (5,10,15 cmH2O), unobstructed breathing, and mild UAO (50 and 200 cmH2O/ml/sec). Inner box represents median value for 10 monkeys, outer Box Inter-Quartile Range (IQR), and bars Non-outlier range. K values from isovolume conditions are statistically significantly higher than those generated using QDC during CPAP, unobstructed breathing (baseline), or mild obstructed breaths (p<0.01). K values using QDC on all conditions of CPAP and unobstructed breathing are similar (p>0.22). K values generated on obstructed breaths of 200 cmH2O/ml/sec are significantly higher than those on CPAP (p<0.02), but not as high as those seen during an isovolume maneuver.
Calibration factors using QDC with inspiratory resistance
K values obtained from QDC calibration with an inspiratory resistance of 50 cmH2O/ml/sec were not different from baseline and CPAP. K values obtained from QDC calibration with an inspiratory resistance of 200 cmH2O/ml/sec were higher than those generated during baseline and CPAP (all p<0.02, Table 1, Figure 4).
Calibration factors and adequacy from Isovolume Maneuver versus QDC
K values generated during Isovolume maneuvers at FRC or end inspiration were significantly higher than those generated under any of the QDC conditions (all p <0.01) (Figure 4). We assessed the accuracy of the K values to calibrate the RIP flow tracings generated from isovolume maneuvers at FRC compared to QDC under all conditions (unobstructed, CPAP 5–15 cmH2O, obstructed breathing 50, 200 cmH2O/ml/sec). With the exception of CPAP of 15 cmH2O, calibration of flow tracings was more accurate with isovolume maneuvers, compared to QDC calibration performed during that condition (p<0.001) (Figure 5). When applying QDC derived K values (from CPAP of 5cmH2O) to RIP flow tracings during complete obstruction (NIF maneuver, when flow should be 0) flow was a median of 193% of the flow seen during tidal breathing (IQR 105,242) at FRC and 206% (IQR 159,301) at IC (Figure 3).
Figure 5. Adequacy of RIP calibration Isovolume versus QDC on different conditions.

RIP calibration compared to gold standard of spirometry using different RIP calibration techniques (Isovolume maneuver with a NIF (IsoCal) versus QDC calibration on the represented value of CPAP, unobstructed breathing, or mild UAO. A. CPAP 15 cmH2O B. CPAP 10 cmH2O C. CPAP 5 cmH2O D. Tidal Breathing E. Inspiratory Resistance 50 cmH2O/ml/sec F. Inspiratory Resistance 200 cmH2O/ml/sec. Bar is median, outer box is interquartile range, and whisker is non-outlier range. Calibration using isovolume conditions resulted in a lower median mean square error than calibration using QDC for all conditions other than CPAP of 15cmH20 (p<0.0001). For CPAP of 15, QDC resulted in lower median mean square error than calibration using isovolume conditions (p<0.001).
Discussion
Using an animal model for pediatric UAO, we have demonstrated both quantitatively and qualitatively that calibration of RIP flow tracings using isovolume maneuvers during a NIF procedure yields nearly identical flow tracings as spirometry. This calibration remains accurate when applied to tidal breathing, and increasing degrees of UAO. There is minimal variability in the flow calibration when the NIF procedure is performed at end exhalation (FRC) compared to end inspiration, making it appropriate to use in a clinical environment, when the NIF procedure may be timed imprecisely in the respiratory cycle. In addition, we have demonstrated that the application of CPAP of 5 cmH2O (which would be typical for a child close to extubation) has minimal impact on the relative contribution of the RC and ABD during tidal breathing, as seen by similar proportionality constants (K values) during QDC Calibration. This allows RIP flow calibration performed on CPAP of 5 cmH2O to be applied to children after extubation. However, in this model, QDC calibration of the RIP flow signal was generally poor, and worsened as UAO increased. As such, an isovolume maneuver is needed for accurate RIP flow signal calibration to detect UAO in these primates.
When translating this model to infants and children, there are several considerations. Physiologic similarities between human infants and children and adult Rhesus monkeys allows this animal model to be appropriate for pediatric UAO. The body habitus and weight (on average 8–11 kilograms), tracheal sizes (17, 19, 20), and lung volumes (standardized to body weight) (21–30) (31) are comparable between human infants and children and adult Rhesus monkeys (Table 2). Moreover they are alike in terms of respiratory system resistance, compliance, oxygen consumption, and minute ventilation (32, 33). The respiratory mechanics for human infants and children more closely parallel those of the adult Rhesus monkey than a human adult. Similar to human infants and children, adult Rhesus monkeys often have a significant contribution of their respiratory work from abdominal muscles, particularly as respiratory load increases. To this end, human infants are more similar to adult Rhesus monkeys, than adult humans. Measures of work of breathing are quite similar between human infants and children and adult Rhesus monkeys, (17, 20, 34, 35), and both infants and monkeys have a characteristic rise in the degree of thoraco-abdominal-asynchrony (TAA) using non-calibrated RIP with UAO (20, 34).
Table 2.
Summary of pertinent similarities and differences in pulmonary mechanics and lung function measurements in human infants, Rhesus monkeys, and Adult Humans
| Test | Tracheal Size (ETTmmID) |
Vital Capacity (ml/kg) |
Resistance (respiratory system) (cmH2O/ml/s) |
Compliance (respiratory system) (ml/cmH2O/kg) |
FRC (ml/kg) |
TLC (ml/kg) |
Phase angle (range -degrees) |
|---|---|---|---|---|---|---|---|
| Species | |||||||
| Human infant | 3.0 – 4.5 | 50 – 60 | 0.04–0.08 | 0.8 – 1.1 | 22 – 25 | 70 – 80 | 10 – 180 |
| Rhesus monkey | 3.0 – 5.0 | 60 – 70 | 0.04–0.08 | 0.8 – 1.1 | 20 – 25 | N/A | 60–150 |
| Adult human | 6.0 – 9.0 | 55 – 60 | 0.01 – 0.03 | 1.2 – 1.5 | 30 – 35 | 80 – 90 | 5 – 70 |
The need for accurate RIP flow calibration necessitates careful consideration of the compliance of the chest wall in these different populations. While some adult studies have demonstrated similar RIP calibration factors between isovolume maneuvers and tidal breathing, the more compliant chest wall of the infant limits direct translation of these findings to children (5, 11). Through our previous observations with the adult Rhesus monkeys, their chest wall is even more complaint than the human infant. In this study, we have demonstrated that isovolume maneuvers using a NIF results in excellent calibration of RIP flow tracings to detect worsening UAO. As such, we believe the same will hold for human infants, given their chest wall compliance lies between these monkeys and adults. What was unclear, however, is if QDC calibration under tidal conditions in infants and children can result in adequate interpretation of flow from RIP, as has been shown in some studies on adult humans. This study has demonstrated that QDC calibration is inadequate in these monkeys, particularly as UAO worsens. This will need to be tested in children to determine if they are more similar to the monkeys, or adult humans. It is likely that this difference is a function of age, as infants are quite different from adolescents.
There are some clear practical points that can be directly translated to children from this model. First, the NIF procedure either at FRC or end-inspiration yields isovolume maneuvers, and is an appropriate way to calibrate the RIP flow tracing, yielding near-identical flow patterns as spirometry. There was stability in the K values generated across 10 consecutive breaths, repeated 3 times, arguing that it can be done in children with fewer breaths (a standardized NIF in children is 3–5 breaths).
Second, if the patient has pre-existing UAO, QDC calibration under conditions of UAO yields quite different calibration factors than those seen under unobstructed breathing or complete obstruction. Therefore, it may not be possible to get accurate calibration of RIP flow in a child who has ongoing obstructed breathing, as would be helpful in a non-intubated child with croup.
There are certainly some limitations with this technique, and our findings. First, one monkey had significant variability in K values during the different isovolume maneuvers. This monkey did breathe with a more rapid shallow breathing pattern, and was obese. While this may have contributed to the variability in these results, it represents the reality that these specific maneuvers may be difficult to perform in a reproducible manner for all children (or monkeys). Second, RIP assumes a two-compartment model of the respiratory system, which may not hold as UAO worsens. While the mean squared error between spirometry and calibrated RIP increased with both techniques (QDC or Isovolume) as inspiratory load increased (perhaps reflecting inadequacies of the two compartment model or non-linearity of the relationship between RC and ABD), RIP calibration during isovolume maneuvers was clinically interpretable for almost all monkeys, with only 2% of observations demonstrating inadequate calibration on qualitative analysis. Hence, even if assumptions of the two compartment model do not fully hold, one can likely still gain enough information from RIP with esophageal manometry to determine flow limitation. Third, and most importantly, these findings would suggest that calibration of RIP must occur during isovolume maneuvers, which limit its applications for small infants and children who are not cooperative, or who are not mechanically ventilated through an endotracheal tube (where one can do a NIF). Future research is needed to determine whether QDC calibration of RIP flow is accurate in children, or for other applications. Our study was primarily interested in RIP flow calibration to detect UAO, where it is logical that isovolume conditions more closely reflect inspiratory airway obstruction. It may be that QDC works adequately for other applications, particularly when there may be more variability of spontaneous inspiratory effort. Finally, these monkeys with minimal pulmonary disease may not fully reflect the respiratory disease of children cared for in intensive care units. However, it is unlikely that this residual pulmonary disease prior to extubation would limit applications of RIP calibration related to post-extubation UAO, because the underlying respiratory disease is unlikely to change dramatically from CPAP of 5 cmH2O prior to extubation to unassisted breathing after extubation.
Despite the limitations, these findings afford the opportunity to use these techniques to study post extubation UAO objectively in children in the intensive care unit. Esophageal catheters, RIP bands, and spirometry through a pneumotach can all be connected to the patient while they are invasively mechanically ventilated through an endotracheal tube. RIP calibration can occur during a NIF procedure to simulate isovolume maneuvers, as well as during 5 minutes of steady state breathing on CPAP of 5 cmH2O. Direct evaluation of calibrated RIP flow using both techniques can be compared to simultaneous measurements of spirometry both during complete obstruction during the NIF and during tidal breathing on CPAP prior to extubation as part of an extubation readiness test (36). If calibration is accurate, then the calibrated RIP signals, combined with the esophageal pressure tracings, can generate flow-pressure loops to quantify objectively whether the patient has UAO after the endotracheal tube is removed. Using this objective outcome measure in a large clinical study should produce more definitive data regarding risk factors for post-extubation UAO, the utility of therapies targeted at post-extubation UAO, and afford the potential for definitive studies on prevention strategies, if a high-risk group of patients can clearly be identified.
Methods
We performed an interventional trial in adult Rhesus monkeys of similar weight and pulmonary development to human infants. We have previously developed and validated techniques for infant pulmonary function testing on similar primates, with many investigations of RIP and respiratory mechanics (17, 20, 26, 31–33). All experiments were performed at the Novartis Animal Care Facility in Basel, Switzerland. The study was approved by the Cantonal Animal Protection Committee and the Institutional Animal Care and Use Committee (IACUC) at Children’s Hospital Los Angeles.
Anesthesia Protocol
We anesthetized monkeys with intramuscular ketamine and continuous intravenous propofol, titrated to achieve spontaneous breathing with minimal movement or response to noxious stimuli. All monkeys were endotracheally intubated with a 4.5 mm ID cuffed endotracheal tube (Rüsch, Teleflex Medical, Bad Liebenzell Germany), with the cuff inflated to occlude any audible air leak.
Monitoring
We placed an esophageal balloon catheter from the mouth into the lower third of the esophagus (Carefusion, Avea SmartCath 8Fr). We confirmed position through a series of complete and partial endotracheal tube occlusions, as previously described (37). We placed Respiratory Inductance Plethysmogrpahy (RIP) bands (Viasys Healthcare, Respiband Plus, Hoechberg, Germany) around the nipples and umbilicus (20). We connected a calibrated pneumotachometer (Viasys Variflex 51000-40094) to the endotracheal tube. Each monkey received continuous monitoring of electrocardiogram, pulse oximetry, end-tidal CO2, temperature, and intermittent non- invasive blood pressure.
Hardware and Software
We connected the esophageal manometer, pneumotachometer, and RIP Bands to the Bicore II (CareFusion, Houten, The Netherlands). We displayed and recorded data (at a frequency of 200Hz) on a laptop computer using Polybench (Applied Biosignals GmbH, Weener, Germany). We then post processed the obtained measurements using VivoSense software (Vivonetics, San Diego, CA).
Study Protocol
We used a variety of different conditions to evaluate the adequacy of calibrated RIP flow signals against spirometry. Specifically, we were interested in the similarity of the flow signals, rather than the absolute value of flow itself. To do RIP flow signal calibration, the basic principle is to determine what proportion of the flow comes from the Ribcage (RC) versus the abdomen (ABD). RIP flow is derived from the time based derivative of RIP volume. The following equation sets the changes in uncalibrated rib cage (ΔµVRC) and uncalibrated abdominal (ΔµVAB) volumes approximately equal to the change in volume at the airway opening (ΔµVao): ΔµVao ≈ M[ΔµVRC + K(ΔµVAB)]. The term K establishes the electrical proportional relationship between the uncalibrated rib cage (ΔµVRC) and uncalibrated abdominal (ΔµVAB) signals from RIP. The term M scales the quantity of [ΔµVRC + K(ΔµVAB)] to the volume measured with spirometry (ΔµVao). Since we were primarily interested in a signal which is proportional to flow (as opposed to actual flow), the M term was not important, and not calculated.
Isovolume calibration-obstructed breaths (NIF procedure)
We performed an isovolume maneuver by occluding the endotracheal tube for 10 consecutive attempted breaths, confirming zero flow with spirometry. We repeated the maneuver 3 times, with several minutes between maneuvers. We initiated the maneuvers at two different lung volumes: FRC (end expiration) and end inspiration during tidal breathing. We calculated K values for each individual breath attempt based on the following equation K = − (ΔµVRC/ ΔµVAB) where ΔµVRC is the change in volume of the Rib Cage compartment during a single breath attempt, and ΔµVRC is the change in volume of the Abdomen compartment during the same breath. We excluded breaths in which isovolume conditions could not be guaranteed (i.e. visible flow on spirometry tracings). We averaged the 10 K values for each maneuver. To evaluate the reproducibility of the technique, we describe the variation in average K values during each of these three maneuvers. For analysis of accuracy of RIP flow signal calibration against spirometry, we used the average of these three values to arrive at the final isovolume K value for each monkey, rounded to one decimal point.
QDC Calibration, tidal breathing
We performed Quantitative Diagnostic Calibration (QDC) using 5 minutes of steady state breathing. Because naturally breathing subjects do not breathe with a constant tidal volume (VT), QDC collects a large number of breaths and excludes those with large deviations (ࣙ1 Standard Deviation) from the mean sum of RIP (as a surrogate for VT). The QDC algorithm calculates K based on the following equation: KQDC = ΣSD(ΔµVRC)/SD(ΔµVAB). Here, SD refers to the standard deviation of the change in ABD and RC volumes over the entire 5 minute period.
We performed a 5 minute QDC calibration under several different conditions: unobstructed tidal breathing, Continuous Positive End Expiratory Pressure (CPAP) of 5, 10 and 15 cmH2O, and obstructed breathing. We obtained CPAP by providing a constant flow of fresh gas into a flow inflating anesthesia bag connected to the endotracheal tube and partially occluded to maintain the desired airway pressure. Inspiratory obstruction was achieved by placing 2 fixed calibrated resistors (50 and 200 cmH2O/ml/sec, Hans Rudolph, Kansas City, MO) at the inspiratory limb of a Y shaped (non-rebreathing) valve attached to the endotracheal tube. This allowed inspiratory resistance, with no added resistance to exhalation.
Simulated Upper Airway Obstruction
To evaluate the accuracy of the different RIP calibration techniques to detect UAO, we applied eleven fixed calibrated resistors (range none-1000 cmH2O/ml/sec) as described above. The order of resistors was random for each monkey, and each resistor was in place for two minutes. We have previously demonstrated that the endotracheal tube itself is not a source of flow limitation, and should therefore not influence the results (19). We gave monkeys three minutes of recovery with unobstructed breathing between resistors, with a longer time if vital signs had not returned to baseline.
Comparison of flow tracings measured with spirometry and calibrated RIP
We compared the flow signal generated from calibrated RIP to the spirometry flow signal both quantitatively and qualitatively. The quantitative approach calculates the Mean Squared Error (MSE) between the calibrated RIP flow signal and the Spirometry signal partitioned as inspiratory or expiratory for each breath. Both spirometry and RIP flow tracings were normalized for each breath (min of zero and max of 1). We then subtracted normalized spirometry flow from normalized RIP flow for time points along the inspiratory and expiratory limb of the breath (at a frequency of 200Hz). Thus, for each breath the MSE during inspiration can be represented as Σ [NormFlowSpir − NormFlowRIP]2/(number of samples during inspiration).
During complete obstruction (when spirometry flow was confirmed zero), we compared the relative flow seen from different RIP calibration methods, to the flow seen during tidal breathing. We computed the percentage difference as the average peak-trough difference of RIP calibrated flow during the isovolume maneuver/the average peak to trough difference of RIP calibrated flow during tidal breathing. Perfect RIP calibration would result in values close to zero.
Qualitative methods were based on visual inspection of two plots: flow/time, and flow/esophageal pressure (Figure 6). We determined the adequacy of calibration of RIP based upon the equivalence of the shape of RIP and Spirometry flow-time plots and the interpretation of flow-limitation from flow-pressure plots (5). Two investigators (RK, RF) independently graded each condition on a scale from 1 (terrible) –5 (excellent) (Figure 6). We adjudicated disagreements to arrive at a consensus interpretation. We felt it important to have both quantitative and qualitative analysis to enable objective mathematical comparison and understand whether clinical interpretation was different.
Figure 6. Qualitative assessment of accuracy of calibrated RIP (right) against spirometry (left).

The top plots are flow versus pressure, examining flow limitation. The bottom tracings are flow versus time, using both techniques.
Outcome measures and analysis
The primary objective was to determine whether isovolume calibration of the RIP flow signal was needed to detect different degrees of inspiratory flow limitation. To simulate the potential application to post-extubation UAO in children, the conditions of interest were: a NIF at FRC (isovolume) versus 5 minutes of tidal breathing on CPAP of 5 cmH2O (QDC). Both conditions could be used in children before extubation. We compared a one minute trend of median MSE between calibrated RIP and spirometry flow on inspiration during steady state breathing, for each resistor level. We also compared the median values of the qualitative adequacy of calibration (scale 1–5) between the two techniques at each resistor level. Wilcoxon Sign Rank Test was used for nonparametric, paired analysis.
Additional outcomes included comparison of median K values generated from: 1. isovolume maneuvers at FRC and end inspiration to evaluate whether normal clinical variability in the NIF maneuver would still yield interpretable results (to examine reproducibility). 2. QDC calibration generated during unobstructed tidal breathing, CPAP of 5, 10, and 15 cmH2O to determine whether CPAP alters K values. 3. QDC calibration generated with unobstructed tidal breathing versus that on 50 and 200 cmH2O/ml/sec of inspiratory resistance, to determine whether QDC calibration could be performed in the setting of ongoing airway obstruction. We compared median K values with Friedman’s ANOVA, to account for repeated measures per monkey, non parametric distribution, and multiple comparisons.
Acknowledgments
The authors would like to sincerely thank Daniel Wyss for all of his hard work and assistance with implementation of the experimental protocol, and Brian Richardson DVM for his support and guidance
Statement of Financial Support: (1) National Institutes of Health/National Institutes of Child Health and Development 1K23HL103785 (RK); United States of America; (2) Novartis Pharmaceutical Grant-in Aid for facilities (CJN); Switzerland
Footnotes
There are no conflicts of interest for any of the authors.
References
- 1.Argent AC, Newth CJ, Klein M. The mechanics of breathing in children with acute severe croup. Intensive Care Med. 2008;34:324–32. doi: 10.1007/s00134-007-0910-x. [DOI] [PubMed] [Google Scholar]
- 2.Newth CJ, Levison H, Bryan AC. The respiratory status of children with croup. J Pediatr. 1972;81:1068–73. doi: 10.1016/s0022-3476(72)80233-6. [DOI] [PubMed] [Google Scholar]
- 3.Khemani R, Schneider J, Morzov R, Markovitz B, Newth CJ. Pediatric Upper Airway Obstruction: Interobserver variability is the road to perdition. J Crit Care. 2013;28:490–7. doi: 10.1016/j.jcrc.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argent AC, Hatherill M, Newth CJ, Klein M. The effect of epinephrine by nebulization on measures of airway obstruction in patients with acute severe croup. Intensive Care Med. 2008;34:138–47. doi: 10.1007/s00134-007-0855-0. 2008. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan V, Zhang JN, Russi EW, Bloch KE. Detection of inspiratory flow limitation during sleep by computer assisted respiratory inductive plethysmography. Eur Respir J. 2000;15:570–8. doi: 10.1034/j.1399-3003.2000.15.24.x. [DOI] [PubMed] [Google Scholar]
- 6.Mayer OH, Clayton RG, Sr, Jawad AF, McDonough JM, Allen JL. Respiratory inductance plethysmography in healthy 3- to 5-year-old children. Chest. 2003;124:1812–9. doi: 10.1378/chest.124.5.1812. [DOI] [PubMed] [Google Scholar]
- 7.Allen JL, Wolfson MR, McDowell K, Shaffer TH. Thoracoabdominal asynchrony in infants with airflow obstruction. Amer Rev of Resp Dis. 1990;141:337–42. doi: 10.1164/ajrccm/141.2.337. [DOI] [PubMed] [Google Scholar]
- 8.Duffty P, Spriet L, Bryan MH, Bryan AC. Respiratory induction plethysmography (Respitrace): an evaluation of its use in the infant. Amer Rev of Resp Dis. 1981;123:542–6. doi: 10.1164/arrd.1981.123.5.542. [DOI] [PubMed] [Google Scholar]
- 9.Stefano JL, Spitzer AR, Baumgart S, Davis JM, Fox WW. Inductive plethysmography–a facilitated postural calibration technique for rapid and accurate tidal volume determination in low birth weight premature newborns. Amer Rev of Resp Dis. 1986;134:1020–4. doi: 10.1164/arrd.1986.134.5.1020. [DOI] [PubMed] [Google Scholar]
- 10.Revow MD, England SJ, Stogryn HA, Wilkes DL. Comparison of calibration methods for respiratory inductive plethysmography in infants. J Appl Physiol. 1987;63:1853–61. doi: 10.1152/jappl.1987.63.5.1853. [DOI] [PubMed] [Google Scholar]
- 11.Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, et al. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol. 1989;66:410–20. doi: 10.1152/jappl.1989.66.1.410. 1989. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa RC, Carvalho CR, Moriya HT, Barbosa RCC, Carvalho CRFd, Moriya HT. Respiratory inductive plethysmography: a comparative study between isovolume maneuver calibration and qualitative diagnostic calibration in healthy volunteers assessed in different positions. Jornal Brasileiro De Pneumologia: Publicacao Oficial Da Sociedade Brasileira De Pneumologia E Tisilogia. 2012;38:194–201. doi: 10.1590/s1806-37132012000200008. [DOI] [PubMed] [Google Scholar]
- 13.De Groote A, Paiva M, Verbandt Y. Mathematical assessment of qualitative diagnostic calibration for respiratory inductive plethysmography. J Appl Physiol. 2001;90:1025–30. doi: 10.1152/jappl.2001.90.3.1025. [DOI] [PubMed] [Google Scholar]
- 14.Stromberg NO. Error analysis of a natural breathing calibration method for respiratory inductive plethysmography. Medical & Biological Engineering & Computing. 2001;39:310–4. doi: 10.1007/BF02345285. [DOI] [PubMed] [Google Scholar]
- 15.Poole KA, Thompson JR, Hallinan HM, Beardsmore CS. Respiratory inductance plethysmography in healthy infants: a comparison of three calibration methods. Eur Respir J. 2000;16:1084–90. doi: 10.1034/j.1399-3003.2000.16f11.x. [DOI] [PubMed] [Google Scholar]
- 16.Adams JA, Zabaleta IA, Stroh D, Johnson P, Sackner MA. Tidal volume measurements in newborns using respiratory inductive plethysmography. Amer Rev of Resp Dis. 1993;148:585–88. doi: 10.1164/ajrccm/148.3.585. 1993. [DOI] [PubMed] [Google Scholar]
- 17.Ross PA, Hammer J, Khemani R, Klein M, Newth CJ, et al. Pressure-rate product and phase angle as measures of acute inspiratory upper airway obstruction in rhesus monkeys. Pediatr Pulmonol. 2010;45:639–44. doi: 10.1002/ppul.21212. [DOI] [PubMed] [Google Scholar]
- 18.Prisk GK, Hammer J, Newth CJL. Techniques for measurement of thoracoabdominal asynchrony. Pediatr Pulmonol. 2002;34:462–72. doi: 10.1002/ppul.10204. [DOI] [PubMed] [Google Scholar]
- 19.Hammer J, Newth CJ. Influence of endotracheal tube diameter on forced deflation flow-volume curves in rhesus monkeys. Eur Respir J. 1997;10:1870–3. doi: 10.1183/09031936.97.10081870. 1997. [DOI] [PubMed] [Google Scholar]
- 20.Hammer J, Newth CJ, Deakers TW. Validation of the phase angle technique as an objective measure of upper airway obstruction. Pediatr Pulmonol. 1995;19:167–73. doi: 10.1002/ppul.1950190305. [DOI] [PubMed] [Google Scholar]
- 21.Hammer J, Patel N, Newth CJ. Effect of forced deflation maneuvers upon measurements of respiratory mechanics in ventilated infants. Intensive Care Med. 2003;29:2004–8. doi: 10.1007/s00134-003-1895-8. [DOI] [PubMed] [Google Scholar]
- 22.Hammer J, Newth CJ. Effort and volume dependence of forced-deflation flow-volume relationships in intubated infants. J Appl Physiol. 1996;80:345–50. doi: 10.1152/jappl.1996.80.1.345. [DOI] [PubMed] [Google Scholar]
- 23.Hammer J, Newth CJ. Infant lung function testing in the intensive care unit. Intensive Care Med. 1995;21:744–52. doi: 10.1007/BF01704742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammer J, Newth CJ. Effect of lung volume on forced expiratory flows during rapid thoracoabdominal compression in infants. J Appl Physiol. 1995;78:1993–7. doi: 10.1152/jappl.1995.78.5.1993. 1995. [DOI] [PubMed] [Google Scholar]
- 25.Hammer J, Patel N, Newth CJ, Hammer J, Patel N, Newth CJL. Effect of forced deflation maneuvers upon measurements of respiratory mechanics in ventilated infants. Intensive Care Med. 2003;29:2004–8. doi: 10.1007/s00134-003-1895-8. [DOI] [PubMed] [Google Scholar]
- 26.Newth CJ, Amsler B, Anderson GP, Morley J. The effects of varying inflation and deflation pressures on the maximal expiratory deflation flow-volume relationship in anesthetized rhesus monkeys. Amer Rev of Resp Dis. 1991;144:807–13. doi: 10.1164/ajrccm/144.4.807. [DOI] [PubMed] [Google Scholar]
- 27.Newth CJ, Enright P, Johnson RL. Multiple-breath nitrogen washout techniques: including measurements with patients on ventilators. Eur Respir J. 1997;10:2174–85. doi: 10.1183/09031936.97.10092174. [DOI] [PubMed] [Google Scholar]
- 28.Schibler A, Hammer J, Isler R, Buess C, Newth CJ. Measurement of lung volume in mechanically ventilated monkeys with an ultrasonic flow meter and the nitrogen washout method. Intensive Care Med. 2003–4;30:127–32. doi: 10.1007/s00134-003-2028-0. [DOI] [PubMed] [Google Scholar]
- 29.Hammer J, Numa A, Newth CJ. Total lung capacity by N2 washout from high and low lung volumes in ventilated infants and children. Amer J of Resp Crit Care Med. 1998;158:526–31. doi: 10.1164/ajrccm.158.2.9710096. [DOI] [PubMed] [Google Scholar]
- 30.Hammer J, Numa A, Newth CJ. Albuterol responsiveness in infants with respiratory failure caused by respiratory syncytial virus infection. J Pediatr. 1995;127:485–90. doi: 10.1016/s0022-3476(95)70088-9. [DOI] [PubMed] [Google Scholar]
- 31.Sivan Y, Hammer J, Newth CJ. Measurement of high lung volumes by nitrogen washout method. J Appl Physiol. 1994;77:1562–4. doi: 10.1152/jappl.1994.77.3.1562. [DOI] [PubMed] [Google Scholar]
- 32.Newth CJ, Amsler B, Anderson GP, Morley J. The ventilatory and oxygen costs in the anesthetized rhesus monkey of inhaling drugs used in the therapy and diagnosis of asthma. Amer Rev of Resp Dis. 1991;143:766–71. doi: 10.1164/ajrccm/143.4_Pt_1.766. [DOI] [PubMed] [Google Scholar]
- 33.Newth CJ, Amsler B, Richardson BP, Hammer J. The effects of bronchodilators on spontaneous ventilation and oxygen consumption in rhesus monkeys. Pediatr Res. 1997;42:157–62. doi: 10.1203/00006450-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Sivan Y, Deakers TW, Newth CJ. Thoracoabdominal asynchrony in acute upper airway obstruction in small children. Amer Rev of Resp Dis. 1990;142:540–4. doi: 10.1164/ajrccm/142.3.540. [DOI] [PubMed] [Google Scholar]
- 35.Willis BC, Graham AS, Yoon E, Wetzel RC, Newth CJL. Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med. 2005;31:1700–5. doi: 10.1007/s00134-005-2821-z. [DOI] [PubMed] [Google Scholar]
- 36.Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, et al. Weaning and extubation readiness in pediatric patients. Pediatric Critical Care Medicine. 2009;10:1–11. doi: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coates AL, Davis GM, Vallinis P, Outerbridge EW. Liquid-filled esophageal catheter for measuring pleural pressure in preterm neonates. J Appl Physiol. 1989;67:889–93. doi: 10.1152/jappl.1989.67.2.889. [DOI] [PubMed] [Google Scholar]


