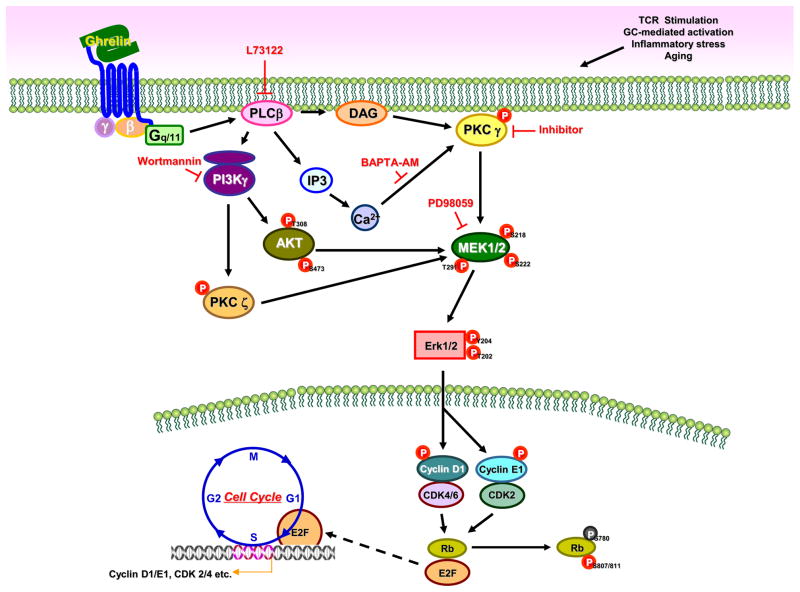
Figure 10. GHS-R1a-mediated ghrelin signaling in murine CD4+ T cells.
Ligation of acylated ghrelin to the GHS-R1a receptor results in the activation of the kinases PI3K/AKT and MAPK, which \ play important roles in T cell activation and proliferation. PLCβ is activated through GHS-R1a signaling via the activation of Gαq associated subunits resulting in the phosphorylation of PI3K and AKT. Phosphorylation of AKT at Thr-473/308 by PI3K results in the activation of protein kinase activity. PLCβ activates protein kinase C (PKC) γ and ζ and induces the phosphorylation of Erk1/2 resulting in the phosphorylation of cyclin D1 and E1, which play an important role in cell cycle progression. AKT also regulate the Erk1/2 and the activation of cyclin D1 and E1 and the downstream cyclin D1 associated kinases (CDKs). Phosphorylation of Ser795 and Ser807/811in Rb is thought to be mediated by the CDK4/6 and CDK2. Rb binds to the E2F-1 transcription factor preventing it from interacting with the cell’s transcription machinery. However, upon phosphorylation, E2F mediates the transactivation of target genes that encode proteins that help to facilitate the G1/S transition and S-phase. Thus, ghrelin receptor signaling influences T cell activation and promotes T cell proliferation directly (as observed with T-cell hybridoma cells) or possibly via costimulatory pathways (as observed with primary CD4+ T cells). Ghrelin may counteract the adverse effects of chronic stress and aging on T cell proliferation and survival.