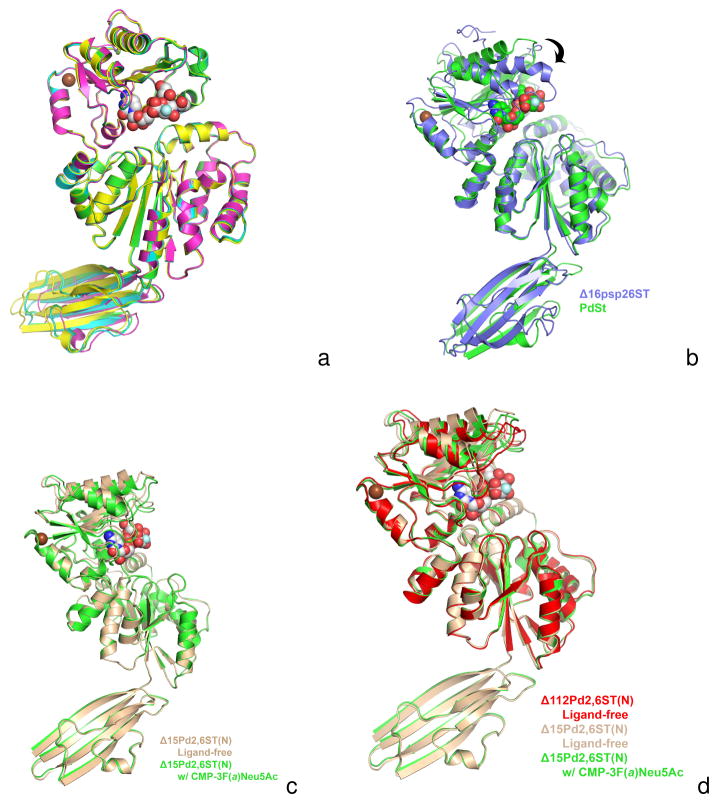
Figure 5.
Figure 5A. A superposition of all four monomers of the CMP-3F(a)Neu5Ac-bound Δ15Pd2,6ST(N) structures seen in the triclinic P1 asymmetric unit. Each monomer is colored differently and only the GT-B folds are aligned, illustrating a small shift in Ig-like domain orientations. The subunits GT-B domains are superimposed with rmsd ranges 0.21–0.32 Å for 374 equivalent α-carbons.
Figure 5B. A superposition of the Δ15Pd2,6ST(N) structure (green) onto V. Photobacterium sp. JT-ISH-224 sialyltransferase (Δ16Psp26ST) (blue). The donor substrate analogue CMP- 3F(a)Neu5Ac bound in Δ15Pd2,6ST(N) is shown in space filling spheres with green-colored carbon atoms. The CMP of the Δ16Psp26ST structure is not shown for clarity, but lies very close to the CMP moiety of CMP-3F(a)Neu5Ac bound in Δ15Pd2,6ST(N). Only the N-terminal Rossmann domains of the GT-B fold are aligned (rmsd 0.95 Å) illustrating the differences in closure around the active site (C-terminal Rossmann domain swings down as shown by arrow) upon binding activated donor. The Δ15Pd2,6ST(N) structure with CMP-3F(a)Neu5Ac bound resembles, more closely, other GT-B sialyltransferases in the ligand-free state (not shown). The alignment also illustrates the different orientations of the Ig-like domains of two structures.
Figure 5C. A superposition between the ligand-free (tan) and CMP-3F(a)Neu5Ac-bound (green) structures of Δ15Pd2,6ST(N). The alignment shows a small closure between the two domains of the GT-B fold upon soaking in CMP-3F(a)Neu5Ac (white-colored carbon spheres). Crystal packing and lattice contacts may prevent the larger closure seen in other GT-B sialyltransferases [2, 18].
Figure 5D. A superposition of the Δ112Pd2,6ST(N) construct lacking the Ig-like domain and substrates (red) and the ligand-free (tan) and CMP-3F(a)Neu5Ac-bound (green) structures of Δ15Pd2,6ST(N). The alignment shows the most closed structure occurs in the ligand free Δ112Pd2,6ST(N). This is likely due to crystal contacts illustrating the inherent flexibility between the two Rossmann-like domains. The CMP-3F(a)Neu5Ac seen in the Δ15Pd2,6ST(N) binary structure is drawn as white-colored carbon spheres.