Summary
This study examined whether physical intimate partner violence (IPV) victimization was associated with diurnal patterns of salivary cortisol in a community sample of 122 couples in their 30s from predominantly lower socioeconomic status backgrounds. Findings indicate that women with higher levels of victimization exhibited flatter patterns of diurnal cortisol characterized by both higher midday levels and more attenuated decreases in cortisol levels across the day, compared to women with lower levels of victimization. However, men's victimization was not associated with their diurnal cortisol levels. This study advances our understanding of the association between physical IPV victimization and dysregulated hypothalamic-pituitary-adrenal (HPA) axis functioning in women, which is likely to have further implications for their subsequent mental and physical health.
Keywords: Couples, Hypothalamic-Pituitary-Adrenal (HPA) Axis, Cortisol, Intimate Partner Violence (IPV), Physical Aggression, Victimization
1. Introduction
Victimization of physical intimate partner violence (IPV), which ranges from being pushed, slapped, or kicked to severely beaten and assaulted with a knife or gun, may have long-lasting physical and psychological health consequences – including anxiety, depression, chronic pain, and psychosomatic disorders (Lawrence et al., 2012). However, despite the high prevalence of IPV (Slep and O'Leary, 2005), the underlying mechanisms that may explain the effects of IPV on health outcomes are not well understood. Growing evidence suggests that the quality of the romantic relationship may influence individuals’ health outcomes through physiological processes including cardiovascular, endocrine, and immune functioning (Robles and Kiecolt-Glaser, 2003). From this perspective, one pathway that links IPV with negative health outcomes may be via the impact of IPV victimization on dysregulation of stress-linked endocrine processes (Repetti et al., 2002), more specifically, alterations in hypothalamic-pituitary-adrenal (HPA) axis activity (Feinberg et al., 2011). Increasing the understanding of the direct associations between IPV and HPA axis activity may help explain individual differences in vulnerabilities to IPV-related health problems, which would facilitate the development of more effective treatment programs (Inslicht et al., 2006). In a community sample of couples, the present study examined associations between physical IPV victimization and diurnal patterns of the glucocorticoid hormone cortisol (as measured in saliva), a major hormonal end product of the HPA axis.
1.1. IPV and HPA axis activity
As a primary component of the stress reactivity and regulation system, the HPA axis releases the adrenocortical steroid hormone cortisol in response to stress, which then activates various systems throughout the brain and body to manage challenges (Sapolsky et al., 2000). Well-regulated cortisol production exhibits a strong circadian rhythm with levels typically peaking 20–30 minutes after waking (i.e., a cortisol awakening response [CAR]), declining rapidly in the next few hours, and more gradually throughout the day until reaching a low point in the late evening (Saxbe et al., 2008). Although activation of the HPA axis is critical to adaptive functioning, chronic or prolonged activation of the system is detrimental for physical, psychosocial, and cognitive functioning (Heim et al., 2000; Sapolsky et al., 2000; Fries et al., 2005; Chrousos, 2009). Chronic stress or psychosocial maladjustment is often associated with “flat” or “blunted” diurnal cortisol patterns with low cortisol levels in the morning without the typical steep nonlinear decline across the day (Fries et al., 2005; Saxbe et al., 2008) – which is in turn linked to a range of poor outcomes such as coronary heart disease and obesity (e.g., Brotman et al., 2007; Ruttle et al., 2013).
HPA axis activity is sensitive to interpersonal stressors (Diamond, 2001; Powers et al., 2006), including conflicts within romantic relationships (Kiecolt-Glaser and Newton, 2001; Heffner et al., 2004). Married couples’ hostile and negative behaviors were associated with increases in cortisol levels (Kiecolt-Glaser et al., 2003; Robles et al., 2006). Recent evidence suggests dysregulation in HPA axis activity among individuals with a history of physical IPV victimization, especially in women (Seedat et al., 2003; Pico-Alfonso et al., 2004; Inslicht et al., 2006). Using plasma cortisol collected once in the morning, Seedat et al. (2003) found that women who were victims of physical IPV showed lower levels of morning cortisol relative to women who were not victims of IPV. Women who were physically abused also showed higher evening salivary cortisol levels compared to women who were not abused, even after controlling for women's age, childhood abuse, and other adulthood victimization history (Pico-Alfonso et al., 2004). Similarly, Johnson and colleagues (2008) found that women's chronic exposure to physical IPV was associated with lower waking cortisol response. However, prior studies on cortisol activity in relation to IPV relied on limited measures of cortisol and IPV and relatively small samples of women recruited through service centers for abused women (e.g., women's resource center). Thus, whether these findings can be generalized to couples in the community is unclear.
1.2. Gender differences in response to IPV
Although few studies have examined gender differences in HPA axis activity in relation to IPV, evidence suggests that the association between IPV and HPA axis activity may differ for men and women. Robles and colleagues (2006) found that negative interaction patterns were related to flatter declines in cortisol for wives only. Similarly, Saxbe and colleagues (2008) found that greater marital satisfaction was associated with diurnal cortisol patterns (higher morning values and a steeper decline across the day) for wives only. Greater physiological reactivity to marital conflict for women compared to men may be partly because of the women's tendency to be more attuned to the emotional quality of marital interactions compared to the husbands (Kiecolt-Glaser and Newton, 2001; Robles et al., 2006; Saxbe et al., 2008). However, most of the existing studies have focused only on women victims, emphasizing the need for further evidence regarding potential gender differences in the HPA axis activity associated with IPV victimization.
1.3. The present study
Using multivariate hierarchical modeling (Raudenbush et al., 1995), we examined associations between physical IPV victimization and men's and women's diurnal patterns of salivary cortisol. Men and women with higher levels of physical victimization were hypothesized to exhibit dysregulated diurnal cortisol patterns, as indexed by (1) lower CAR – defined as 30-minute post-awakening cortisol levels minus awakening cortisol levels; (2) higher midday cortisol levels; (3) less linear decline in midday cortisol levels; and (4) low and flat cortisol levels across the day, without the typical steep nonlinear decline than those with lower levels of physical victimization. The present study extends previous work by investigating (1) whether findings from prior research would generalize to a community sample of couples from predominantly lower socioeconomic status (SES) backgrounds; (2) multiple parameters of diurnal cortisol patterns by modeling intra-individual variability and inter-individual differences among couples; (3) effects of physical IPV controlling for psychological IPV (e.g., yelling, insulting, and threatening behavior) and other factors that have been found to be associated with diurnal cortisol patterns (i.e., women's employment, number of children, and relationship satisfaction); and (4) gender differences in the associations between IPV and HPA axis activity.
2. Methods
2.1. Participants
Data for the present study were from the Oregon Youth Study (OYS)-Couples Study. The men were originally recruited to the OYS through fourth-grade classes (ages 9-10 years) from public schools in a midsized Pacific Northwest city that had higher-than-average incidences of juvenile delinquency in their neighborhoods (N = 206, participation rate = 74%). The men have been almost annually assessed over the past 30 years. The men's parents were predominantly of lower SES; approximately 50% of the men had juvenile arrest records and only 52% graduated from high school with their class. When the men were ages 17-19 years, the OYS-Couples Study was initiated to examine these men's adjustment with their romantic partners. To date, the men and their partners have participated in eight couples’ assessments from late adolescence (T1, ages 17-19 years) through early adulthood (T8, ages 35-36 years). The present study focused on the T8 assessment in which couples completed salivary cortisol sample collections.
Of 145 couples who participated at the T8 assessment, 127 couples had at least 1 partner who contributed to the saliva collection. Of these 127 couples, 5 couples were excluded because they were same-sex (n = 2), pregnant (n = 2), or broke up just prior to the saliva collection (n = 1). Note that same-sex couples were not included in the present analyses in order to test gender differences within male-female couples. Data were further excluded due to one of the partners reporting diabetes (n = 1), or flu-like symptoms during the collection (n = 1), or having had incomplete information on the study predictors (n = 6). This resulted in a total of 122 couples for the analysis – with complete data on both partners for 109 couples and partial data for 13 couples. Couples’ demographic characteristics are presented in Table 1. On average, men and women who were included in the present analyses were not significantly different from those who were excluded on any of the demographic variables or study predictors, except for women's ethnic/racial background; women who were included in the analyses were more likely to be of an ethnic/racial minority (16%) than excluded women (0% ethnic/racial minority; χ2 [1] = 4.12, p = .04).
Table 1.
Sample descriptive statistics.
| Men | Women | Couples | |
|---|---|---|---|
| Age (years) | 36.33 (.58) | 34.00 (6.22) | 35.17 (3.12) |
| Ethnic/racial minority (n, %) | 13 (11%) | 19 (16%) | n/a |
| Income (per $10,000) | 4.42 (3.71) | 1.81 (1.96) | 6.22 (4.39) |
| Education (n, %) | |||
| Less than 12 years | 22 (18%) | 23 (19%) | n/a |
| High school graduate (regular, diploma program or GED) | 25 (21%) | 69 (57%) | n/a |
| Greater than 12 years | 75 (61%) | 29 (24%) | n/a |
| Relationship status (n, %) | |||
| Dating/other | n/a | n/a | 17 (14%) |
| Cohabitating | n/a | n/a | 32 (26%) |
| Married | n/a | n/a | 73 (60%) |
| Relationship length (years) | n/a | n/a | 8.84 (6.37) |
| Prevalence of psychological IPV victimization (n, %) | 107 (88%) | 105 (86%) | n/a |
| Psychological IPV victimization | .80 (.72) | .76 (.75) | .78 (.72) |
| Prevalence of physical IPV victimization (n, %) | 22 (18%) | 13 (11%) | n/a |
| Physical IPV victimization | .04 (.12) | .04 (.20) | .04 (.15) |
| Cortisol (ug/dL) | |||
| Upon awakening | .30 (.19) | .31 (.17) | n/a |
| 30-minutes post-awakening | .34 (.22) | .37 (.23) | n/a |
| Midday | .12 (.16) | .12 (.13) | n/a |
| Before bed | .11 (.16) | .11 (.18) | n/a |
| Women's full-time employment in last year (Months) | n/a | 6.26 (4.81) | n/a |
| Number of children in household fulltime (n) | n/a | n/a | 1.75 (1.29) |
| Relationship satisfaction | 111.84 (18.80) | 108.27 (19.35) | 110.06 (17.32) |
Note: Tabled numbers denote the mean followed by the (standard deviation) unless noted otherwise.
2.2. Procedures
Each partner completed in-person interviews and questionnaires independently and participated in problem-solving discussion tasks together in the laboratory at the Oregon Social Learning Center (OSLC). Assessments lasted approximately 3–3.5 hr. During the problem-solving tasks, six saliva samples were collected, thus familiarizing couples with the saliva collection procedure. Those who agreed to participate in the daily salivary cortisol collections were asked to take saliva collection kits home, along with a daily diary to record information about the collections. Trained assessors reviewed the protocol with the participants verbally and also gave them a copy of the written protocol. Couples were instructed to take four saliva samples per day (upon awakening, 30 minutes post awakening, in the mid to late afternoon, and at bedtime) across four consecutive work days (yielding 16 maximum samples per person). Samples were collected by passive drool into 1.7ml Eppendorf tubes. Couples were specifically instructed not to eat, drink, or brush their teeth before sample collections. Couples were also instructed to record any deviations from these guidelines, general health, medication use, wake and bed time, and saliva collection times on the daily diary each day. Participants received a call the evening before sampling began and were reminded of key aspects of the saliva collection protocol. Each vial was pre-labeled by the research staff with the date and sample number. Participants were instructed to refrigerate all saliva samples at home until the end of the collection and then either mail the samples in prepaid envelopes or drop them off at OSLC. Samples were then refrigerated for a maximum of a week onsite until they were sent to the Snodgrass Lab at the University of Oregon and frozen at -80C until assayed. All study procedures were approved by the Institutional Review Board at OSLC.
2.3. Measures
2.3.1. Salivary cortisol
Saliva collections were assessed in duplicate using a commercially available enzyme immunoassay kit (1-3002; Salimetrics, State College, PA) for salivary cortisol following the manufacturer's recommended protocol. This immunoassay kit was designed and validated for the quantitative measurement of cortisol in saliva and has been extensively used in research; additional information is available from Salimetrics (http://www.salimetrics.com/assets/documents/1-3002.pdf). Eighty-four percent of the samples assayed in duplicate met the coefficient of variation (CV) and/or absolute value difference criterion suggested by Salimetrics. Duplicates for the remaining 16% of the sample varied by more than 15% and thus were re-assayed. The test used 25 ul of saliva, had a lower limit of sensitivity of .007 ug/dL, and a range of sensitivity from .007 to 3.0 ug/dL. The average intra-assay and inter-assay coefficients across four days was 10.57% and 10%. Cortisol samples that were contaminated with food or blood were eliminated from the assay. In addition, thirty-seven cortisol samples (< 1%) were treated as missing due to cortisol values exceeding 2 ug/dL (n = 6) or the participants reporting having brushed their teeth, used nicotine, and/or imbibed caffeinated beverages or alcohol just prior to their saliva collections (n = 31). This yielded a total of 3532 cortisol samples to be used in the current analyses. On average, individuals provided saliva samples at 14.5 of the 16 total possible collections. The average of the duplicate tests was used in the analyses. Cortisol units are expressed in micrograms per deciliter (ug/dL).
2.3.2. Physical IPV victimization
Men's and women's physical IPV victimization in the past year was assessed using the physical assault subscales of the Conflict Tactics Scale–Revised (CTS2, Straus et al., 1996). Each partner reported both on his/her victimization and on their partner's victimization on a seven-point scale (0 = “never or less than once a year” to 6 = “daily”). The physical assault subscales included six items each (e.g., I/my partner pushed or shoved me/my partner). Internal reliabilities of self- and partner reports of physical IPV ranged from .66 to .84. For both men's and women's victimization scores, self- and partner's reports were significantly associated (r = .27, p < .001and r = .84, p < .001 for men's and women's victimization, respectively). To limit biases associated with monosource reporting, we averaged the men's self-reports and the partner reports on men's victimization and, similarly, the women's self-reports and partner reports on women's victimization for subsequent analyses.
2.3.3. Control variables
To take into account potential influences on diurnal cortisol patterns, psychological IPV victimization, women's employment, the number of children living in the household full time, and couples’ relationship satisfaction were included in the analyses as control variables (Adam and Gunnar, 2001).
2.3.3.1. Psychological IPV
Psychological IPV victimization was assessed by the psychological aggression subscales from the CTS2 (described above). The subscales included five items each (e.g., I/my partner shouted or yelled at me/my partner). Self- and partner reports had good internal reliabilities ranging from α =.72 to α = .86. In addition, both self- and partner reports were significantly associated (r = .60, p < .001 for men's and r = .59, p < .001 for women's psychological victimization). Similar to physical IPV victimization, self- and partner reports on psychological victimization were averaged for the subsequent analyses.
2.3.3.2. Women's employment
Women's employment was assessed using four questions: “How many months out of the past 12 months have you worked for pay: (1) full time (35 or more hr/wk); (2) part time (6 to 34 hr/wk); (3) very part time (1 to 5 hr/wk); and (4) occasional odd jobs, but no regular hours?” A weighted sum denoting the number of months women were employed full time in the last year (ranging from 0 to 12 months) was then calculated.
2.3.3.3. Number of children in the household
Participants were asked how many biological, step, and/or adopted children they had and whether these children lived with them full or part time. The maximum number of children living in the home full time reported by both partners was then used in the analyses.
2.3.3.4. Relationship satisfaction
Both partners reported on their relationship satisfaction using the Dyadic Adjustment Scale (Spanier, 1976; e.g., “How often do you discuss or have you considered divorce, separation, or terminating your relationship”). Response scales for the items ranged from 0 (always disagree) to 5 (always agree), except for one item that was coded on a 0 to 4 scale, yielding a total score range of 0–139 (α = .90 for men and .92 for women).
2.4. Data analytic plan
Dependence among (1) individuals’ repeated cortisol samples (i.e., four collections per day across four consecutive days) and (2) cortisol levels across partners were accounted for by employing multivariate hierarchical modeling (Raudenbush et al., 1995). One of the major advantages of this approach is that key parameters describing intra-individual variability in diurnal cortisol rhythms (e.g., CAR, slope of diurnal cortisol rhythms) are simultaneously estimated and can be predicted by inter-individual differences among couples (e.g., physical IPV). Estimates of individuals’ diurnal cortisol patterns that are indicative of their overall basal HPA-axis functioning (rather than day-specific diurnal patterns) were obtained by modeling the four cortisol collections per day across the four consecutive days at Level 1 (within-couples), while simultaneously allowing for mean differences in couples’ cortisol levels across repeated collection days (Adam, 2006). The Level-1 model for the ith couple may be written as follows:1
| (1) |
SR Cortisoli denotes the square root of the ith couples’ cortisol level (i.e., the transformed outcome variable). Male and Female denote “0/1” gender indicator variables. Thus, lines 2–4 of Equation 1 denote men's effects and lines 5–7 denote women's effects. By employing this multivariate modeling approach, the Level-1 equation summarizes data at the couple rather than individual level; thus, line 8, errori, denotes variability in couples’ cortisol levels that is not explained by the model. Regression coefficients with an “i” subscript were modeled as random effects, those without an “i” subscript denote fixed effects. Variation in cortisol levels across repeated collection days was accounted for in lines 4 and 7. Parameters βm7i, – βm9i for men and βf7i, – βf9i for women denote systematic mean variation among couples’ cortisol levels on the first collection day compared to the other three collection days. They were modeled with three contrast coefficients: Day2, Day3, and Day4 coded as “1” to denote the second, third, and fourth collection days, respectively, and “0” otherwise. Individuals were aligned on the same Time metric by using their hours since waking, which was person-mean centered for each day around awakening and final collection times. Lines 3 and 6 in Equation 1 account for differences in individuals’ awakening times, days of collections, and behaviors on the days of collections (e.g., medication use, alcohol and nicotine use, and exercise). Specifically, variation between participants’ awakening times was controlled for by entering the number of hours difference between awakening times and noon (i.e., Hours Since Noon, HSN in Equation 1), which was grand-mean centered at approximately 4.5 hours before noon (i.e., average awakening time = 7:30 am).2 Variation in the days of week that participants provided the saliva samples was accounted for by using the Weekend contrast coefficient that equaled “0” for the weekdays (Monday through Friday) and “1” for the weekends (Saturday and Sunday). A binary Flag variable indicates whether or not individuals had taken non-steroidal medications (e.g., depression/anxiety medication), imbibed alcohol, used nicotine, and/or exercised on the day(s) of their saliva collections, thus accounting for any potential variation in cortisol levels attributable to these factors known to influence HPA axis activity.3
The regression coefficients in lines 2 and 5 of Equation 1 denote the primary effects of interest, characterizing individuals’ diurnal cortisol patterns according to their: (1) midday levels of cortisol on the first day (intercepts βm0i and βf0i), (2) rates of linear change in cortisol levels at midday (slopes βm1i and βf1i), (3) amount of curvature or dampening (i.e., nonlinear change) in cortisol levels across the entire day (quadratic effects βm2i and βf2i), and (4) CAR (CARs βm3i and βf3i). CARs were defined as the difference in cortisol levels at the 30-minute post-awakening collection (coded as “0.5”) minus the awakening collection (coded as “-0.5” or “0” otherwise) (Adam and Kumari, 2009). Partners’ midday cortisol levels (intercepts βm0i and βf0i) and rates of change in cortisol levels at midday (slopes βm1i and βf1i) were free to correlate. In order to examine if differences in the four diurnal cortisol parameters would be predicted by individuals’ levels of physical IPV victimization, the Level-2 (between-couples) model included:
| (2) |
while simultaneously controlling for mean differences in cortisol levels across repeated collection days. (Note: Similar equations were used for women, where women's physical IPV victimization predicted women's cortisol parameters).
The γ's denote the fixed (i.e., average) effects, and the b's denote the random variance effects (i.e., variability around the average). Phy. IPV denotes the average level of men's or women's physical IPV victimization reported by both partners in the last year. The regression coefficients preceding these predictor variables (γm0,phy – γm3,phy for men [andγf0,phy – γf3,phy for women, not shown]) capture the associations between inter-individual differences in men's or women's physical IPV victimization and intra-individual differences in their diurnal cortisol patterns. Gender differences were examined by constraining the effects of physical IPV on all four of the diurnal cortisol parameters to be equal for men and women (γm0,phy = γf0,phy , ..., γm3,phy= γf3,phy), and then change in overall model fit when all of the parameters that were allowed to vary were examined using the Scaled χ2 difference test (Satorra and Bentler, 2011). Finally, the Level-2 equation was expanded to include four control variables – psychological IPV victimization, the number of children living in the home full-time, women's months of full-time employment over the last year, and each partner's relationship satisfaction. All of the Level-2 predictors were grand-mean centered by gender except for the number of children living in the home full time, which was grand-mean centered by couples.
| (3) |
(Note: Similar equations were used for women, where control variables predicted their cortisol parameters.)
3. Results
3.1. Descriptive
Bivariate correlations among the Level-2 (between-couples) predictors and men's and women's cortisol values for each of the four collection times (averaged across four days) are shown in Table 2. All of the IPV measures were positively associated with one another and negatively associated with men's and women's relationship satisfaction. Moreover, higher levels of women's, but not men's, physical IPV victimization were significantly associated with higher levels of both men's and women's midday and evening cortisol values and with lower levels of women's 30-minute post-awakening cortisol values. Finally, fewer hours of women's employment were associated with higher levels of women's physical IPV victimization and more children living in the home full time.
Table 2.
Bivariate correlations among men's and women's Cortisol values (by collection time) and the study predictors.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol: | ||||||||||||
| 1. Awakening | -- | .51*** | .10 | .14* | .09 | .06 | .07 | .10 | .03 | −.03 | .05 | −.01 |
| 2. 30-min post-awakening | .53*** | -- | .09 | .09 | .03 | .05 | .05 | .11 | .09 | .00 | .07 | −.03 |
| 3. Midday | .11M | .05 | -- | .49*** | .09 | .09 | .14** | .12 | −.10 | −.12M | −.002 | −.07 |
| 4. Evening | .17** | .09 | .49*** | -- | .13 | .14 | .16** | .14 | −.08 | −.10 | −.02 | −.12 |
| 5. Women's psychological IPV victimization | −.002 | −.01 | .03 | .07 | -- | .90*** | .41*** | .53*** | −.05 | −.02 | −.54*** | −.50*** |
| 6. Men's psychological IPV victimization | −.01 | −.04 | .06 | .06 | .90*** | -- | .46** | .64*** | −.09 | .00 | −.60*** | −.59*** |
| 7. Women's physical IPV victimization | −.04 | −.05M | .15* | .13* | .41*** | .46** | -- | .76*** | −.16* | −.06 | −.31 | −.34*** |
| 8. Men's physical IPV victimization | −.02 | −.04 | .11 | .09 | .53*** | .64*** | .76*** | -- | −.19* | .03 | −.36*** | −.42*** |
| 9. Women's employment | .08 | .13M | −.13M | −.02 | −.05 | −.09 | −.16* | −.19* | -- | −.22** | .02 | .05 |
| 10. Number of children | .08 | .06 | −.04 | .00 | −.02 | .00 | −.06 | .03 | −.22** | -- | −.04 | −.05 |
| 11. Men's relationship satisfaction | −.01 | .10 | .04 | .00 | −.54*** | −.60*** | −.31*** | −.36*** | .02 | −.04 | -- | .65*** |
| 12. Women's relationship satisfaction | .05 | .07 | −.10 | −.09 | −.50*** | −.59*** | −.34*** | −.42*** | .05 | −.05 | .65*** | -- |
Note: Values above and below the diagonal for the cortisol variables (first four rows and columns) denote correlations with men and women, respectively. Cortisol values were square-root-transformed prior to calculation of correlation coefficients. Standard errors of the correlation coefficients were estimated using a sandwich estimator to account for the dependence among repeated observations from the same men and women across their four consecutive days of saliva collections.
p < .001.
p < .01.
p < .05.
p < .10.
3.2. Diurnal cortisol patterns
The unconditional model (Table 3) indicated that men's and women's average cortisol levels 30 minutes after waking were significantly higher than their cortisol levels upon awakening. Likewise, both men and women had midday cortisol levels significantly greater than zero, significant linear decreases in cortisol levels at midday, and significant non-linear change or dampening in cortisol levels over the course of the day. All eight of the random effects defining variability around men's and women's average diurnal cortisol patterns (i.e., CARs, intercepts, slopes, and quadratic effects) were significant, except for men's random quadratic effect that was marginally significant. On average, men's and women's second, third, and fourth midday cortisol levels were not significantly higher or lower than their midday cortisol levels on the first day of collections. Similarly, variability in men's and women's cortisol levels on the first day of collections did not significantly differ from the variability on any of the other three collection days, except for women's cortisol levels on the fourth day. Individuals’ awakening times, whether the collections occurred on a weekday versus weekend, and the flag variable denoting medication use, alcohol and nicotine use, and exercise were not significant for men's and women's cortisol levels.
Table 3.
Effects of physical IPV victimization on diurnal patterns of cortisol.
| Unconditional Model | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| Fixed Effect | Men | Women | Men | Women | Men | Women |
| Cortisol (midday) | ||||||
| Intercept | .38*** | .36*** | .37*** | .36*** | .38*** | .36*** |
| Physical IPV victimization | -- | -- | .25* | .08** | .23M | .09** |
| Psychological IPV victimization | -- | -- | -- | -- | .01 | −.01 |
| Women's employment | -- | -- | -- | -- | −.001 | .00 |
| Number of children | -- | -- | -- | -- | −.01 | −.01 |
| Relationship satisfaction | -- | -- | -- | -- | .00 | .00 |
| Hours since awakening (slope) | ||||||
| Intercept | −.34*** | −.39*** | −.34*** | −.40*** | −.34*** | −.39*** |
| Physical IPV victimization | -- | -- | .03 | .17*** | −.10 | .14** |
| Psychological IPV victimization | -- | -- | -- | -- | .01 | −.02 |
| Women's employment | -- | -- | -- | -- | −.01* | −.003 |
| Number of children | -- | -- | -- | -- | −.03* | −.02 |
| Relationship satisfaction | -- | -- | -- | -- | −.001 | −.002M |
| Hours since awakening2 (quadratic) | ||||||
| Intercept | .41*** | .50*** | .42*** | .52*** | .42*** | .51*** |
| Physical IPV Victimization | -- | -- | −.13 | −.49*** | −.26 | −.39** |
| Psychological IPV Victimization | -- | -- | -- | -- | .10 | .10 |
| Women's employment | -- | -- | -- | -- | .01 | .02 |
| Number of children | -- | -- | -- | -- | .06 | .09M |
| Relationship satisfaction | -- | -- | -- | -- | .002 | .004 |
| Cortisol awakening response (CAR) | ||||||
| Intercept | .05*** | .07*** | .05*** | .08*** | .05*** | .08*** |
| Physical IPV Victimization | -- | -- | .04 | −.05* | .06 | −.04 |
| Psychological IPV Victimization | -- | -- | -- | -- | .01 | .01 |
| Women's employment | -- | -- | -- | -- | .01* | .003 |
| Number of children | -- | -- | -- | -- | .01 | .001 |
| Relationship satisfaction | -- | -- | -- | -- | .001 | .00 |
| Collection day (mean difference test) | ||||||
| Day 2 versus Day 1 | −.01 | −.01 | −.004 | −.01 | −.004 | −.01 |
| Day 3 versus Day 1 | −.01 | −.01 | −.01 | −.01 | −.01 | −.01 |
| Day 4 versus Day 1 | .01 | .01 | .01 | .01 | .01 | .01 |
| Hours since noon | .08 | .07 | .08 | .08 | .08 | .09 |
| Weekend | −.01 | −.01 | −.01 | −.01 | −.01 | −.01 |
| Flag variable | −.007 | .01 | −.01 | .01 | −.01 | .01 |
| Random Effect | ||||||
| Level-1 variance | ||||||
| Couples’ residual | .02*** | -- | .02*** | -- | .02*** | -- |
| Level-2 variances | ||||||
| Cortisol intercept | .01** | .01*** | .01** | .01*** | .01*** | .01** |
| Hours since awakening | .05*** | .04*** | .05*** | .04*** | .04*** | .04*** |
| Hours since awakening2 | .10M | .14** | .09M | .13* | .09M | .11* |
| Cortisol awakening response (CAR) | .01* | .004* | .01* | .004* | .01* | .004* |
| Collection day (variance test) | ||||||
| Day 2 versus Day 1 | .002 | .00 | .002 | .00 | .002 | .00 |
| Day 3 versus Day 1 | .001 | .00 | .001 | .00 | .001 | .00 |
| Day 4 versus Day 1 | .002 | .005** | .002 | .01** | .002 | .01** |
| Level-2 covariances | ||||||
| Couples’ intercepts | .003* | -- | .003* | -- | .003* | -- |
| Couples’ slopes | .02*** | -- | .02*** | -- | .02*** | -- |
| Model fit: | ||||||
| Log likelihood (# estimated parameters) | 1789.43 (37) | 1797.96 (45) | 1812.48 (77) | |||
| Information criteria | ||||||
| AIC | −3504.86 | −3505.92 | −3470.97 | |||
| BIC | −3276.59 | −3228.30 | −2995.93 | |||
| Sample size adjusted BIC | −3394.16 | −3371.29 | −3240.60 | |||
Note. Men's and women's cortisol parameters were predicted by their psychological and physical IPV victimization scores. Hours since noon = number of hours difference between individuals’ awakening times and noon. Information criteria and effects pertaining to couples (i.e., the Level-1 residual variance and Level-2 covariances) are reported in the Men's column only.
p < .001.
p < .01.
p < .05.
p < .10.
3.3. Physical IPV victimization and diurnal cortisol patterns
Next, we examined whether differences between individuals’ diurnal cortisol patterns were associated with the level of men's and women's physical IPV victimization over the last year. First, overall model fit was significantly improved by allowing the effects of physical IPV on all four of the diurnal cortisol parameters to differ for men and women (Scaled χ2 difference [4] = 11.87, p = .018). Likewise, the addition of physical IPV (Table 3, Model I) yielded a significant improvement in overall model fit over the unconditional model (Scaled χ2 difference [8] = 29.19, p < .001). Men with higher levels of physical IPV victimization – compared to men with lower levels of physical IPV victimization – had significantly higher midday cortisol levels (intercepts). No significant effects of physical IPV victimization emerged for men's CARs, rates of change in cortisol at midday (slopes), and nonlinear changes in cortisol levels across the day (quadratic). On the other hand, women with higher levels of physical victimization – compared to women with lower levels of physical victimization – had significantly lower CARs, higher midday levels of cortisol, less linear decline in cortisol at midday, and less nonlinear dampening of cortisol levels over the course of the day. 4
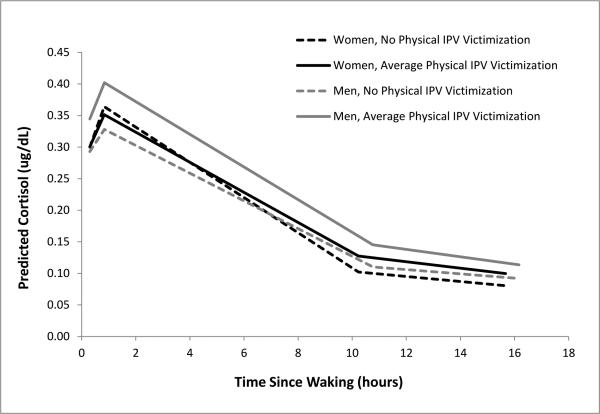
Including the control variables (Table 3, Model II) did not alter the significant effects of women's physical IPV victimization on their diurnal cortisol parameters, and the significance of all other fixed and random effects remained unchanged from Model I. The only exceptions were that the effects of physical IPV on men's midday cortisol levels and women's CARs were attenuated in the presence of control variables. Greater linear decreases in men's midday cortisol levels (i.e., less flattened slopes) were observed for men who had more children and had partners who worked more hours in the past year, compared to men who had fewer children and partners who worked less. Women's employment was also positively related to CARs for men, but none of the control variables were significantly related to the women's diurnal cortisol parameters. Model II did not yield a significant improvement in overall model fit over Model 1 (Scaled χ2difference [32] = 30.61, p = .54). Figure 1 depicts men's and women's predicted diurnal cortisol patterns, given the men's and women's levels of physical IPV victimization for the fully adjusted model (Model II).5
Figure 1.
Men's and women's predicted diurnal cortisol patterns given their experiences of physical IPV victimization (Table 2, Model II).
4. Discussion
Although there has been a great deal of interest in the association between aggression and dysregulated HPA axis activity in children (e.g., Fisher et al., 2007; Bruce et al., 2009), relatively little is known about physiological processes through which IPV exerts its deleterious effects on one's health (Feinberg et al., 2011). Given the high prevalence of IPV among couples and subsequent adjustment problems (Kim et al., 2008), the lack of research on this issue is especially of concern (Feinberg et al., 2011). The present study sought to address this issue by examining the extent to which physical IPV victimization was associated with diurnal cortisol patterns in a community sample of couples in their 30s from predominantly lower SES backgrounds. Overall, the findings indicated that women with higher levels of physical victimization showed significantly higher levels of cortisol in midday and less linear decline across the day, suggesting flatter cortisol rhythms relative to women with lower levels of victimization. Furthermore, the effects of physical victimization were robust in the presence of other control variables.
The finding that women with higher levels of victimization showed flatter diurnal cortisol patterns is in line with findings on the link between marital functioning – specifically marital conflict and IPV, and cortisol activity (e.g., Adam and Gunnar, 2001; Pico-Alfonso et al., 2004; Saxbe et al., 2008). It is interesting to note that women's CARs did not seem to be influenced by physical IPV victimization. This indicates that the flatter cortisol patterns may be largely due to more attenuated decreases in midday and evening levels. This finding is consistent with evidence that showed a group difference in the evening cortisol levels – with women who were physically abused exhibiting higher evening salivary cortisol levels relative to women who were not abused – but no group difference in morning cortisol levels (Pico-Alfonso et al., 2004). However, our finding is somewhat inconsistent with other studies that suggest a negative association between IPV victimization and morning cortisol levels (Seedat et al., 2003; Johnson et al., 2008). Such inconsistencies may be partly due to the community sample of couples employed in the present study, rather than women recruited through service centers for battered women (e.g., shelters). There is considerable evidence that women who enter such agencies exhibit unique characteristics (e.g., high levels of PTSD and depression), compared with the broader population of women involved in IPV experiences in the community (Galano et al., 2013). It should also be noted that, although the present study employed multivariate modeling techniques to simultaneously estimate multiple aspects of diurnal cortisol patterns (e.g., level versus change in level across the day) and had a number of controls, some factors that have been linked to the CAR (e.g., physical/psychiatric conditions, sleep quality and duration) (Fries et al., 2009) were not controlled for, and this may have contributed to the inconsistent results. The impact of IPV victimization on the CAR definitely warrants further research.
It is also worth noting that psychological victimization was not related to any aspects of women's diurnal cortisol patterns. This is somewhat unexpected given the evidence on negative consequences of psychological victimization in the literature. Psychological IPV usually occurs in tandem with physical IPV (Hines and Saudino, 2003) and is associated with a number of detrimental outcomes in women – including psychosocial adjustment (e.g., depression and substance use), poor physical health (e.g., chronic pain), and poor cognitive functioning (see Lawrence et al., 2012). In a preliminary analysis, we examined univariate effects of psychological victimization and found that none of the parameters of diurnal cortisol rhythms were influenced by psychological victimization for either men or women. Many of the existing studies did not consider both types of IPV or did not differentiate types of IPV (Coker et al., 2002); thus, deleterious effects of psychological IPV victimization may have been somewhat confounded in those studies. It is also possible that the pervasiveness of psychological IPV in the relationships of the couples in the present sample (Kim et al., 2008) have desensitized the couples to coercive interaction patterns, rendering a non-significant association between psychological IPV and diurnal cortisol patterns. In prior work on the same group of men in their 20s, we found that over 70% of the men reported psychological aggression in their relationships, and this prevalence rate did not significantly change over time (Kim et al., 2008; Shortt et al., 2012). In the present study, 88% of the men and 86% of the women reported psychological IPV victimization, suggesting that prevalence of psychological IPV remains fairly high among at-risk couples in the mid-30s. Because physical IPV often co-occurs with psychological IPV, it may be difficult to examine unique influences of psychological IPV victimization on HPA axis activity.
Despite the fact that men had higher prevalence rates of physical victimization than did women (Table 1), men's physical victimization was not related to their diurnal cortisol patterns. It is possible that the lack of association for men may have been affected by the low convergence between couples’ reports on physical IPV victimization for men, as reported previously. However, this finding is indeed in line with studies that have shown the quality of marital relationships (e.g., marital satisfaction) play a more significant role for women's diurnal cortisol patterns than for men's (e.g., Saxbe et al., 2008). It also fits with epidemiological findings in the literature that men tend to benefit more from being married but women's health outcomes are more closely associated with the quality of their relationships than with just being married (Kiecolt-Glaser and Newton, 2001). Nonetheless, little is known about associations between victimization and diurnal cortisol for men. Further investigation of gender as a moderator of associations between victimization and diurnal cortisol is warranted.
The finding that physical victimization is associated to diurnal cortisol patterns for women may also be viewed as in line with the existing findings that IPV victimization has more deleterious health consequences for women than for men (Coker et al., 2002; Lawrence et al., 2012). Research has consistently indicated that the involvement in physical IPV is associated with a greater burden for women; women with IPV in their relationships are more likely to visit medical health professionals, be diagnosed with psychiatric disorders such as anxiety or depression, and to report higher levels of stress. Stronger effects of IPV victimization on women's diurnal cortisol patterns may indicate that such victimization has a stronger detrimental effect on the body's adaptation to stress or allostatic load for women than for men (Saxbe et al., 2008). Although the present study did not examine subsequent health outcomes due to HPA axis dysregulation, our findings thus suggest that one of the ways that physical IPV victimization can lead to negative health outcomes such as depression and anxiety in women may be via dysregulation of the HPA axis system (Pico-Alfonso et al., 2004; Inslicht et al., 2006). This also suggests that focusing on endocrine processes such as the stress-linked HPA axis system may further advance our understanding of gender-specific vulnerabilities to IPV-related health outcomes.
Although relationship satisfaction was negatively associated with IPV victimization for both men and women, it was not associated with diurnal cortisol for either men or women (see Table 2). This is inconsistent with findings from a study involving a small sample of middle-class dually employed parents in which higher levels of women's, but not men's marital satisfaction were associated with higher morning cortisol values and steeper cortisol declines over the day (Saxbe et al., 2008). However, differences in findings may reflect demographic differences in study samples. The limited research available on the role of relationship satisfaction in associations between victimization and diurnal cortisol patterns points to an area for further research. It is also not clear why the number of children and the number of hours that the partner worked were significantly associated with men's diurnal slopes only, and this warrants further research.
4.1. Limitations
The present study involved multilevel modeling of cortisol trajectories, which has several strengths (Hruschka et al., 2005; Adam, 2006; Adam and Kumari, 2009). Nonetheless, it is important to note that causal inferences are limited due to the cross-sectional nature of the present study (i.e., data on IPV and diurnal cortisol patterns were taken from a single assessment). As such, we cannot rule out the possibility that HPA axis activity influenced the involvement of physical victimization in couples. As suggested by Feinberg et al. (2011) and others (e.g., Shoal et al., 2003; Shirtcliff et al., 2005), individuals with dysregulated diurnal patterns of cortisol (i.e., flatter diurnal patterns) may be more likely to use physical violence against their partners in conflictual situations (Romero- Martínez et al., 2013; Romero- Martínez et al., 2014). Alternatively, it is possible that the association between IPV involvement and HPA axis activity is bidirectional, leading to reciprocity between the two processes (Granger et al., 1996). In addition, some factors that have been shown to be relevant contributors to dysregulated HPA axis activity were not controlled for, such as chronicity of IPV involvement (e.g., Johnson et al., 2008), PTSD (e.g., Inslicht, et al., 2006), social isolation (e.g., Grant et al., 2009), physical health and sleep quality and duration, and chronic stress other than exposure to IPV (e.g., poverty) (Fries et al., 2009). Likewise, although we incorporated information from the participants’ daily diary into the models, we were unable to monitor wake time and saliva-collection times electronically. It is also possible that some participants from highly conflictual relationships or with hectic lives were also less compliant with the saliva protocol. In addition, given that our measure of CAR was limited, the nonsignificant effect of IPV victimization on women's CAR should be interpreted with caution. Likewise, gender differences in the effects of physical victimization on diurnal cortisol patterns appear to have relatively small associations and thus replication is needed. Finally, because of the limited sample size (N = 122), we were unable to control for multiple comparisons; thus, the results should be interpreted with caution given the possible inflation in the Type I error rate due to multiple hypotheses tests. Furthermore, the limited ethnic/racial diversity (primarily European Americans) and generally low levels of physical IPV victimization in the present sample also should be noted.
Despite these limitations, findings from the present study provide additional empirical support for the proposed link between the quality of romantic relationships and endocrine processes (Robles and Kiecolt-Glaser, 2003; Robles et al., 2006) and, more specifically, for the association between physical IPV victimization on diurnal cortisol patterns in couples. Furthermore, the present study uniquely contributes to the literature by examining both partners in the dyad rather than women only. To our knowledge, the present study is one of the first to examine effects of physical IPV victimization on men's and women's diurnal cortisol patterns in a community sample of couples. Furthermore, we examined multiple indicators of diurnal cortisol patterns by taking advantage of salivary cortisol samples collected four times a day across four consecutive days.
Physical victimization within romantic relationships can be chronic and is likely an ongoing stressor for some couples (Johnson et al., 2008; Kim et al., 2008). There is evidence that dysregulated diurnal cortisol rhythms are related to several physical and psychiatric disorders, including depression (Vrshek-Schallhorn et al., 2013), and that women tend to experience more detrimental effects of IPV victimization than do men (e.g., Ansara and Hindin, 2011). The finding on the significant effects of physical victimization on women's flatter diurnal cortisol patterns provides critical insights into a potential mechanism that may explain gender-specific vulnerability (e.g., depression) in response to IPV. Future research should examine whether such flatter diurnal cortisol patterns have significant implications for women's psychological and physical health. Research on same-sex couples would also help further illuminate gender differences in the effects of IPV victimization on endocrine processes and subsequent health outcomes. In addition, given recent studies on associations among testosterone levels, immune functioning, and cortisol levels among male IPV perpetrators, future research should include a focus on the interplay between the immune and endocrine networks as predictors as well as outcomes of IPV victimization and perpetration in both men and women (Soler et al., 2000; Montoya et al., 2012; Romero-Martinez et al., 2013; Romero-Martinez et al., 2014).
Highlights.
Effects of physical victimization on diurnal patterns of cortisol were examined.
Victimization and cortisol data in a community sample of couples were analyzed.
Women with higher levels of victimization showed flatter diurnal cortisol patterns.
Men's victimization was not associated with their diurnal cortisol levels.
Acknowledgements
Funding for this study was provided by awards from the National Institutes of Health (NIH), U.S. PHS Award Number R01 DA 015485 from the National Institute of Drug Abuse (NIDA); 1R01AA018669 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA); HD 46364 from the National Institute of Child Health and Development (NICHD); and P50DA035763 from the Division of Epidemiology, Services and Prevention Research, NIDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIDA, NIAAA, or NICHD. NIH, NIDA, NIAAA, or NICHD had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Elizabeth A. Streeter for her help with salivary cortisol assay, Jane Wilson and the data collection staff for their commitment to high-quality data, and Sally Schwader for editorial assistance.
Acknowledgements
Funding for this study was provided by awards from the National Institutes of Health (NIH), U.S. PHS Award Number R01 DA 015485 from the National Institute of Drug Abuse (NIDA); 1R01AA018669 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA); HD 46364 from the National Institute of Child Health and Development (NICHD); and P50DA035763 from the Division of Epidemiology, Services and Prevention Research, NIDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIDA, NIAAA, or NICHD. NIH, NIDA, NIAAA, or NICHD had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Elizabeth A. Streeter for her help with salivary cortisol assay, Jane Wilson and the data collection staff for their commitment to high-quality data, and Sally Schwader for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors Capaldi and Kim designed the original study and wrote the protocol. Authors Kim, Tiberio, Capaldi, and Shortt made contributions to the conceptualization of the study. Author Kim reviewed the analyses and wrote the draft of the manuscript. Author Tiberio conducted all of the analyses. Author Squires performed cortisol assays, and author Snodgrass oversaw the lab procedures. All authors reviewed the final manuscript and approved the final manuscript.
For simplicity, only one subscript is shown in all equations, denoting the ith couple; subscripts denoting multiple days and collections are not shown.
To improve model convergence, the differences between the degrees of magnitude of the outcome and the three time-predictor variables in Equation 1 (i.e., Time and Time,2 which denote individuals’ saliva collection times as hours since waking, and HSN, which denotes the difference in hours between awakening times and noon) were rescaled by dividing the three time-predictor variables by a factor of 20 (Muthén and Muthén, 1998-2012).
None of the participants reported the use of steroid mediation; thus it was not included in the flag variable. Separate examination of each behavior – use of depression/anxiety medication (reported by 11% of women and 7% of men), use of other mediations (reported by 26% of women and 16% of men), alcohol use (reported by 40% of women and 47% of men), nicotine use (reported by 34% of women and 42% of men), and exercise (reported by 47% of women and 34% of men) – in the multivariate hierarchical models as a time-dependent dichotomous predictor revealed that none of these indicators were significantly related to cortisol levels, except for women's alcohol use; women showed significant increases in cortisol levels on the days in which they had (vs. had not) consumed alcohol.
We performed an omnibus test to examine the joint significance of effects of physical IPV on men's and women's cortisol slopes and quadratic parameters. Specifically, we fitted a null model that allowed for only main effects of physical IPV on men's and women's cortisol at midday (i.e., intercepts) and then compared the change in overall model fit to an alternative model that allowed for additional effects of physical IPV on men's and women's cortisol slopes and quadratic parameters. The significant Scaled χ2 difference [4] of 12.52, p = .014 suggests significant improvement of overall model fit for the alternative model. This confirms significant effects of physical IPV victimization on cortisol slopes and quadratic parameters.
We examined the correlation between men's and women's level-1 residuals by using the multivariate approach. Specifically, we fitted simultaneous latent growth models for men's and women's diurnal cortisol patterns while also allowing for correlated residuals at level-1. Results indicated that the residuals were indeed significantly related (covariance = .001, p = .032), although allowing for level-1 correlated residuals between men and women did not alter the overall pattern of significance.
Conflict of Interest
There are no potential financial and other conflicts of interest related to the submitted manuscript for all of the authors.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ansara DL, Hindin MJ. Psychosocial consequences of intimate partner violence for women and men. Canda. J. Interpers. Violence. 2011;26:1628–1645. doi: 10.1177/0886260510370600. [DOI] [PubMed] [Google Scholar]
- Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Dev. Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Knoble NB, Shortt JW, Kim HK. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231–280. doi: 10.1891/1946-6560.3.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Coker AL, Davis KE, Arias I, Desai S, Sanderson M, Brandt HM, Smith PH. Physical and mental health effects of intimate partner violence for men and women. Am. J. Prev. Med. 2002;23:260–268. doi: 10.1016/s0749-3797(02)00514-7. [DOI] [PubMed] [Google Scholar]
- Diamond L. Contributions of psychophysiology to research on adult attachment: review and recommendations. Pers. Soc. Psychol. Rev. 2001;5:276–295. [Google Scholar]
- Feinberg ME, Jones DE, Granger DA, Bontempo D. Relation of intimate partner violence to salivary cortisol among couples expecting a first child. Aggressive Behav. 2011;37:492–502. doi: 10.1002/ab.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int. J. Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Galano MM, Hunter EC, Howell KH, Miller LE, Graham-Bermann SA. Predicting shelter residence in women experiencing recent intimate partner violence. Violence Against Women. 2013;19:518–535. doi: 10.1177/1077801213487056. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC, Douglas P. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Dev. 1996;67:3250–3262. [PubMed] [Google Scholar]
- Grant N, Hamer M, Steptoe A. Social isolation and stress-related cardiovascular, lipid, and cortisol responses. An. Behav. Med. 2009;37:29–37. doi: 10.1007/s12160-009-9081-z. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Kiecolt–Glaser JK, Loving TJ, Glaser R, Malarkey WB. Spousal support satisfaction as a modifier of physiological responses to marital conflict in younger and older couples. J. Behav. Med. 2004;27:233–254. doi: 10.1023/b:jobm.0000028497.79129.ad. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress–related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hines DA, Saudino KJ. Gender differences in psychological, physical, and sexual aggression among college students using the Revised Conflict Tactics Scales. Violence Vict. 2003;18:197–217. doi: 10.1891/vivi.2003.18.2.197. [DOI] [PubMed] [Google Scholar]
- Hruschka D, Kohrt B, Worthman CA. Estimating between and within–individual variation in cortisol using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, McCaslin SE, Larkin GL, Hyman KB, Baum A. Increased cortisol in women with intimate partner violence–related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Delahanty DL, Pinna K. The cortisol awakening response as a function of PTSD severity and abuse chronicity in sheltered battered women. J. Anxiety Disord. 2008;22:793–800. doi: 10.1016/j.janxdis.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: newlyweds' stress hormones foreshadow relationship changes. J. Consult. Clin. Psycho. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- Kiecolt–Glaser JK, Newton T. Marriage and health: His and hers. Psychol. Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kim HK, Laurent HK, Capaldi DM, Feingold A. Men's aggression toward women: a 10–year panel study. J. Marriage Fam. 2008;70:1169–1187. doi: 10.1111/j.1741-3737.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence E, Orengo–Aguayo R, Langer A, Brock RL. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406–428. [Google Scholar]
- Montoya ER, Terburg D, Bos PA, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv. Emot. 2012;36:65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. 7th ed. Muthén & Muthén; Los Angeles: 1998-2012. [Google Scholar]
- Pico-Alfonso MA, Garcia-Linares MI, Celda-Navarro N, Herbert J, Martinez M. Changed in cortisol and dehyroepiandrosterone in women victims of physical and psychological intimate partner violence. Biol. Psychiatry. 2004;56:233–240. doi: 10.1016/j.biopsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Powers SI, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples’ attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. J. Pers. Soc. Psychol. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Brennan RT, Barnett RC. A multivariate hierarchical model for studying psychological change within couples. J. Fam. Psychol. 1995;9:161–174. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol. Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiol. Behav. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Robles TF, Shaffer VA, Malarkey WB, Kiecolt-Glaser JK. Positive behaviors during marital conflict: Influences on stress hormones. J. Soc. Pers. Relat. 2006;23:305–325. [Google Scholar]
- Romero-Martínez Á, González-Bono E, Lila M, Moya-Albiol L. Testosterone/cortisol ratio in response to acute stress: a possible marker of risk for marital violence. Soc. Neurosci. 2013;8:240–247. doi: 10.1080/17470919.2013.772072. [DOI] [PubMed] [Google Scholar]
- Romero-Martínez Á, Lila M, Conchell R, González-Bono E, Moya-Albiol L. Immunoglobulin A response to acute stress in intimate partner violence perpetrators: the role of anger expression-out and testosterone. Bio Psychology. 2014;96:66–71. doi: 10.1016/j.biopsycho.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Javaras KN, Klein MH, Armstrong JM, Burk LR, Essex MJ. Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. J. Adolesc. Health. 2013;52:731–737. doi: 10.1016/j.jadohealth.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero M, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler P. Scaling corrections for statistics in covariance structure analysis. University of California Los Angeles: Department of Statistics; Los Angeles, CA: 2011. Retrieved from: http://escholarship.org/uc/item/8dv7p2hr. [Google Scholar]
- Saxbe DE, Repetti RL, Nishina A. Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychol. 2008;27:15–25. doi: 10.1037/0278-6133.27.1.15. [DOI] [PubMed] [Google Scholar]
- Seedat S, Stein MB, Kennedy CM, Hauger RL. Plasma cortisol and neuropeptide Y in female victims of intimate partner violence. Psychoneuroendocrinology. 2003;28:796–808. doi: 10.1016/s0306-4530(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behaviors problems in youth. Dev. Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5–year longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry. 2003;42:1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Shortt JW, Capaldi DM, Kim HK, Kerr DCR, Owen LD, Feingold A. Stability of intimate partner violence by men across 12 years in young adulthood: Effects of relationship transitions. Prev. Sci. 2012;13:360–369. doi: 10.1007/s11121-011-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep AMS, O'Leary SG. Parent and partner violence in families with young children: Rates, patterns, and connections. J. Consul. Clin. Psych. 2005;73:435–444. doi: 10.1037/0022-006X.73.3.435. [DOI] [PubMed] [Google Scholar]
- Soler H, Vinayak P, Quadagno D. Biosocial aspects of domestic violence. Psychoneuroendocrinology. 2000;25:721–739. doi: 10.1016/s0306-4530(00)00022-6. [DOI] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J. Marriage Fam. 1976;38:15–28. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised conflict tactics scales (CTS2): Development and preliminary psychometric data. J. Fam. Issue. 1996;17:283–316. [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow–up and effect of depression history. Psychol. Med. 2013;43:483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]