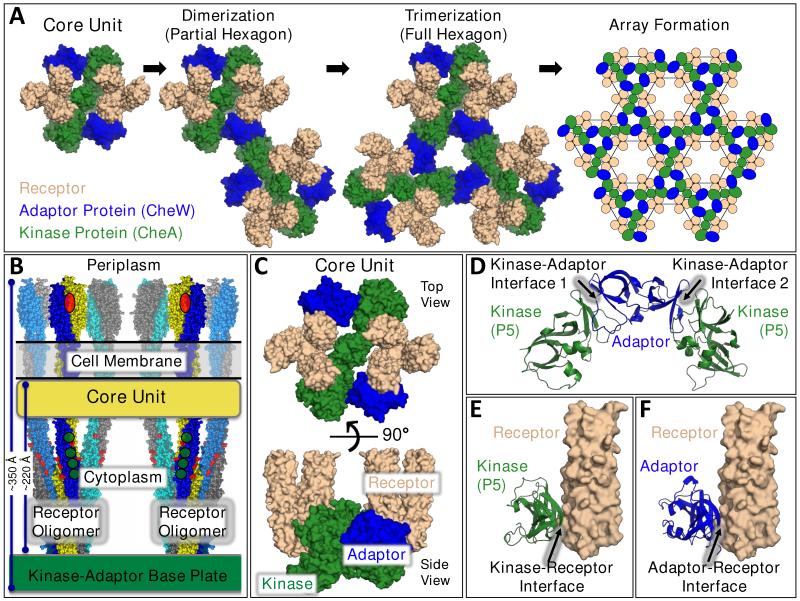
Figure 1. Chemosensory Array Components, Assembly, Architecture, and Stabilizing Contacts.
A) A core unit (far left) is comprised of the three core components: two receptor oligomers (each a trimer-of-homodimers, tan), a homodimeric His-kinase (CheA, green), and two copies of a monomeric adaptor protein (CheW, blue) (28-31,56,63). Core units are hypothesized to associate, forming dimers and then trimers during assembly of individual hexagons (1). Continued assembly forms a hexagonal array (far right) with receptor oligomers located at the vertices (21-23,26,27). In this array, the kinase and adaptor proteins are arranged in a system of interconnecting rings that stabilize individual hexagons and, more globally, the full lattice. In each hexameric ring, the structurally homologous kinase regulatory domain (P5) and the adaptor protein alternate, yielding pseudo-6-fold symmetry with contacts to each of the six surrounding receptor oligomers. Signals can be transmitted within individual core units, or between core units via the kinase-adaptor ring system. B) Schematic side view of the core unit, showing the two receptor trimers-of-dimers, the periplasmic attractant binding sites (red ovals), the cell membrane, the cytoplasmic adaptation sites (green circles), and the kinase-adaptor base plate region (4,23,26,27,46). C) Top view (from periplasm, upper) and side view (lower) of the molecular core unit model, focusing on the receptor protein interaction region and the kinase and adaptor proteins that stably bind to this region (21-23,26,27). D) Current models for the two types of interfaces, 1 and 2, between the kinase regulatory domain (P5) and the adaptor protein in the kinase-adaptor ring system (22,23,26,27). E), F) Current models for the kinase-receptor and adaptor-receptor interfaces, respectively (21,23,26,27).