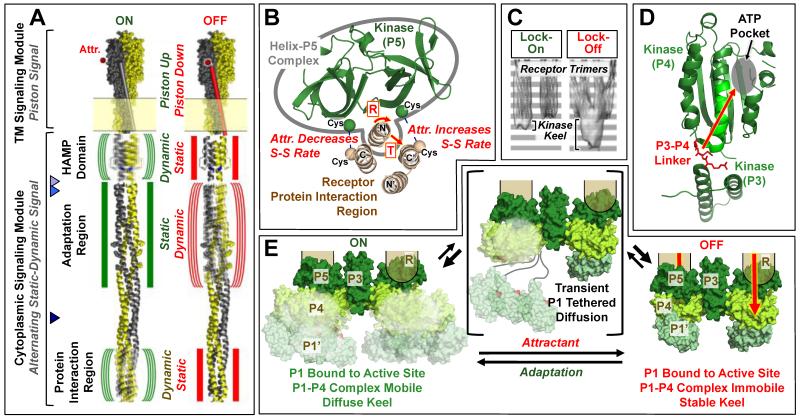
Figure 2. Models of signal transduction in the membrane-bound core unit.
A) Schematic chemoreceptor on- and off-states (green and red symbols, respectively), shown for simplicity in a single chemoreceptor homodimer by omitting the other two dimers of the receptor oligomer. The transmembrane and cytoplasmic signaling modules signal via an established piston mechanism (4,46) and a hypothesized alternating static-dynamic mechanism, respectively (42,43,48-52). The transmembrane signal begins in the periplasm where attractant binds to the apo-receptor and displaces the transmembrane signaling helix (TM2) towards the cytoplasm, thereby driving the piston-up, kinase-activating state into the piston-down, kinase-inhibiting state. The cytoplasmic HAMP domain converts this piston signal into a static-dynamic signal, such that the piston-up, kinase-on state possesses greater HAMP dynamics, while the piston-down, kinase-off state possesses less HAMP dynamics. The model further proposes that in the adaptation region the static-dynamic states are reversed in polarity relative to HAMP, and another polarity reversal is postulated in the protein interaction region. Triangles point to two proposed hinge regions: the major proteolysis site (53) and the nearby hotspot Gly (54) (light and medium blue, respectively), and the multi-Gly hinge (55) (dark blue). B) Shown is the tight complex formed between the kinase regulatory domain (P5) and the N-terminal helix of the receptor protein interaction region (see also Fig. 1E), as well as the attractant-triggered displacement of this complex (either a rotation or translation) detected by disulfide mapping in functional core complexes (21,23). This displacement is believed to transmit, at least in part, the on-off switching signal from the receptor to the kinase. C) Cryo-EM average density maps for signaling arrays containing a lock-on (I241E) or lock-off (A413T) mutant receptor, respectively (40). Within the average core unit, the two receptor trimers of dimers are easily observed, and an additional “keel” of protein density below the base plate region is less evident in locked-on arrays than in locked-off arrays. D) Shown is a hypothesized Class I (see text) allosteric on-off signal, based on NMR studies of CheA kinase, transmitted from the linker between the dimerization (P3) and catalytic (P4) domains, through the catalytic domain to the active site (39). E) Three-state picture of kinase on-off switching compatible with Class I and II models (see text). In Class I, the substrate domain (P1’) of one subunit is proposed to be bound much of the time to the active site on the catalytic domain of the other subunit (P4) awaiting trans-autophosphorylation, yielding a stable P1’-P4 complex regulated by an allosteric on-off signal traveling through the kinase to the P4 active site (28,67). In Class II, the substrate domain P1’ is bound most of the time either to the active site or to a separate inhibitory site on P4 in the on- and off-states, respectively, such that on-off signals regulate the ratio of binding to the two sites (64). In both Class I and II models, the keel domains including the P1’-P4 complex are proposed to be more mobile in the on-state than in the off-state, explaining the low keel density of the on-state in cryo-EM (see (C)) (40). Loss of mobility in the off-state could involve additional constraints on inter-domain linkers, or steric hindrance due to changes in the relative positions of kinase, adaptor and receptor regions. In both models, tethered diffusion of the substrate domain via the long P1-P2 and/or P2-P3 linkers could occur as shown, but would likely be transient to explain the known proteolytic protection of the linkers (10,11,34).