Abstract
Objective
Pharmacokinetics of norethindrone in combination oral contraceptive regimen are well described among HIV+ women treated with ritonavir boosted protease inhibitor therapies; however such characterization is lacking in women using progestin-only contraception. Our objective is to characterize pharmacokinetics of norethindrone in HIV+ women using ritonavir boosted atazanavir treatment during progestin-only contraceptive regimens.
Study Design
An open-label, prospective, non-randomized trial to characterize the pharmacokinetics of norethindrone in HIV+ women receiving ritonavir boosted atazanavir (n=10;treatment group) and other antiretroviral therapy known to not alter norethindrone levels (n=17;control group) was conducted. Following informed consent, women were instructed to take a single daily fixed oral dose of 0.35 mg norethindrone and 300mg/100mg atazanavir/ritonavir for 22 days. On day 22 serial blood samples were collected by venous catheter at 0, 1, 2, 3, 4, 6, 8, 12, 24, 48, and 72 hours. Whole blood was processed to collect serum and stored at −20°C until later analysis using radioimmunoassay. Pharmacokinetic parameters were estimated using non-compartmental method.
Results
In the treatment group, compared to the control group, an increase in area under the curve0-24 (16.69hr*ng/mL vs. 25.20hr*ng/mL; p<0.05) and maximum serum concentration (2.09ng/mL vs. 3.19ng/mL; p<0.05), decrease (25-40%) in apparent volume of distribution and apparent clearance, and unaltered half-life were observed.
Conclusion(s)
Our findings suggest that progestin-only contraceptives, unlike combination oral contraceptives, benefit from drug-drug interaction and achieve higher levels of exposure. Further studies are needed to establish whether pharmacokinetic interaction leads to favorable clinical outcomes.
Keywords: Ritonavir, Norethindrone, Progestin only contraception, Pharmacokinetics
1. INTRODUCTION
Worldwide the leading cause of death among women 18-45 years of age is HIV/AIDS (1). Prevention of mother to child transmission of HIV through contraception and the prevention of unintended pregnancy is a United Nation's millennium development goal for 2010-15 (2). However, hormonal contraceptive use among HIV+ women poses a significant concern for potential drug-drug interaction. Integral to highly active antiretroviral therapy are protease inhibitors which are potent cytochrome P4503A4 (CYP3A4) inhibitors (3). Ritonavir is popularly used as a booster protease inhibitor to enhance therapeutic properties of co-administered protease inhibitors (4).
Norethindrone, a progestin, is typically prescribed as a progestin-only pill or as part of a combined oral contraceptive. Norethindrone is metabolized to M1 metabolite in liver, and to a lesser degree in small intestine, by CYP3A4 predominantly (5). Given the inhibition of CYP3A4 by protease inhibitors, one would speculate elevation of blood levels of norethindrone and thus greater contraceptive efficacy of hormonal contraception. On the contrary, the co-prescription of norethindrone with ritonavir is of limited use in HIV+ women due to the World Health Organization's category-3 designation for the combination of ritonavir and progestin-only pills which implies that the use of the method is not usually recommended unless other appropriate methods are not available or acceptable (6).
Scant data is available to mechanistically understand the interaction between ritonavir and norethindrone in HIV+ women. In women using a combination oral contraceptive, ritonavir was shown to reduce the pharmacokinetic measures of drug exposure (7). Reduction in plasma levels of both estrogenic (ethinyl estradiol) and progestin (norethindrone) were reported; and this potentially explains the category-3 designation by the World Health Organization. Ethinyl estradiol is metabolized by phase 1 (CYP) and phase 2 (uridine glucuronyl transferases) enzymes, both of which are affected by ritonavir. An induction of uridine glucuronyl transferases and/or CYPs by ritonavir would explain the reduction in ethinyl estradiol levels (8). Although ritonavir is both inducer and inhibitor of CYP3A4, the overall effect of ritonavir on chronic administration is inhibition of CYP3A4 (9). This suggests that reduction in ethinyl estradiol levels is most possibly likely due to induction of phase 2 metabolism. On the other, CYP mediated metabolism is the primary route of elimination for norethindrone, and hence reduction in norethindrone levels is counterintuitive given the CYP inhibition of ritonavir. One possible explanation is that the pharmacokinetics of most progestins, including norethindrone, is modulated by plasma levels of estrogenic agent (10). Therefore, reduction in norethindrone levels is likely a function of changes in ethinyl estradiol levels imparted by ritonavir treatment.
The World Health Organization recommendation for progestin only contraceptives was based on the limited data available from combination oral contraceptives (see Annex-1 of the World Health Organization report (6). However, progestin-only contraceptives, unlike combination oral contraceptives, should be devoid of pharmacokinetic alterations induced by estrogenic agent. We hypothesize that ritonavir boosted protease inhibitor regimens will increase the plasma levels of norethindrone resulting in a higher efficacy of progestin-only contraception. In this study, pharmacokinetics of norethindrone was compared in HIV+ women with or without ritonavir boosted atazanavir. Boosted protease inhibitor regimens have reduced the incidence of resistance to protease inhibitor therapies and improved dosing regimen via less frequent dosing. Ritonavir boosted Atazanavir regimen is widely used for the once a day regimen (and therefore potentially higher adherence) and lesser gastrointestinal side-effects (11) and more cost-effective (12) than other protease inhibitors.
2. METHODS & MATERIALS
2.1 Study Design
We conducted an open-label, prospective, non-randomized trial to characterize the steady-state pharmacokinetics of serum norethindrone in HIV+ women receiving ritonavir boosted atazanavir (n=10; treatment group) and other antiretroviral therapy known to not alter norethindrone levels (n=17; control group). The study subjects were HIV+, ovulating, 18-44 years old women with no recent use of hormonal contraception (no more than 30 and 180 days for oral contraceptives and depot medroxyprogesterone acetate, respectively), not taking other CYP3A4 interacting drugs and foods, and agreed to non-hormonal contraception.
The study was approved by University of Southern California Institutional Review Board. Following screening and informed consent, women received a 28-day blister pack of Jolivette® (norethindrone 0.35mg, Watson Pharmaceuticals Inc., Parsippany, NJ) and 22-day supply of Reyataz® (atazanavir 300mg and ritonavir 100mg, Bristol-Myers Squibb company, Princeton, NJ); and instructed to take a one tablet of each at same time of the day for 21 days. Study participants were screened by research staff to identify any barriers to their strict adherence to antiretrovirals as well as norethindrone. An adherence contract was signed, instructed to keep a daily log ingestions of both drugs, and research staff regularly placed phone calls to query about side effects and adherence. On day 22 each woman was admitted to the Clinical Trials Unit, where a clinician observed her final ingestion of both drugs. Serial blood samples were collected by venous catheter at 0 (baseline), 1, 2, 3, 4, 6, 8, 12, 24, 48, and 72 hours. Whole blood was processed to collect serum and stored at −20°C until analyzed.
2.2 Bioanalysis
Norethindrone was quantified in serum by radioimmunoassay as described previously (13). Prior to radioimmunoassay, norethindrone was extracted with ethyl acetate: hexane (3:2) and then purified by Celite column partition chromatography. It was eluted off the column in 20% ethyl acetate in isooctane. Procedural losses were followed by adding small amounts of high specific activity tritiated internal standard (3H-norethindrone) to the serum prior to the extraction step. A highly specific antiserum was used in conjunction with an iodinated radioligand in the radioimmunoassay. Separation of unbound from antiserum-bound norethindrone was achieved by use of second antibody. The sensitivity of the norethindrone radioimmunoassay was 0.06 ng/ml. Intraassay and interassay coefficients of variation range from 4-7% and 9-12%, respectively.
2.3 Pharmacokinetic Analysis
Norethindrone pharmacokinetic data were analyzed by noncompartmental methods using Phoenix WinNonlin (Pharsight, St. Louis, MO, USA). Maximum and minimum serum norethindrone concentrations (Cmax and C24, respectively) and time to maximum concentration (Tmax) were observed values. Area under the curve (AUC) was calculated from time 0 to 24 (AUC0-24) and 0 to 72 h (AUC0–72) using the linear trapezoidal rule and then extrapolated to infinity (AUC0–∞). Drug elimination half-life (t1/2), apparent oral clearance (CL/F) and apparent volume of distribution (Vd/F) were generated using standard PK calculations (t1/2=0.693/λz, where λz is the terminal elimination rate constant; CL/F=dose/AUC0–∞; VD/F=CL/λz). The relative bioavailability (Frelative) of norethindrone in treatment group, compared to control group was calculated as follows: Frelative = (AUC0–∞, treatment) / (AUC0–∞,control).
2.4 Data Analysis
Data were tested for equal variance and normal distribution. Student's t-test was used to assess statistical differences in pharmacokinetic parameters of norethindrone in the control and treatment groups of women. All statistical tests were conducted at α=0.05 using Sigma Plot software (v 11.0; Systat Software, Inc., San Jose, CA).
3. RESULTS
The study participants in both groups are similar in age (control, 37.7 ± 4.6 years (mean ± SD); treatment, 37.9 ± 8.7) and body mass index (control, 29.2 ± 5.7 kg/m2; treatment, 25.8 ± 5.2) (Table 1). Majority of women in both groups are Latinos. The viral load (CD4 count) is also similar between control group (671 ± 234 cells/mm3) and treatment group (663 ± 277).
Table 1.
Study subjects demographics.
| Control (n = 17) | Treatment (n =10) | |
|---|---|---|
| Age (years) | 37.7 (4.6) a | 37.9 (8.7) |
| BMI (kg/m2) | 29.2 (5.7) | 25.8 (5.2) |
| Ethnicity (n): | ||
| Latino | 9 | 7 |
| Black | 4 | 2 |
| Asian | 1 | 1 |
| Caucasian | 3 | 0 |
| Viral load (CD4 cells/mm3) | 671 (234) | 663 (277) |
data represents mean (standard deviation)
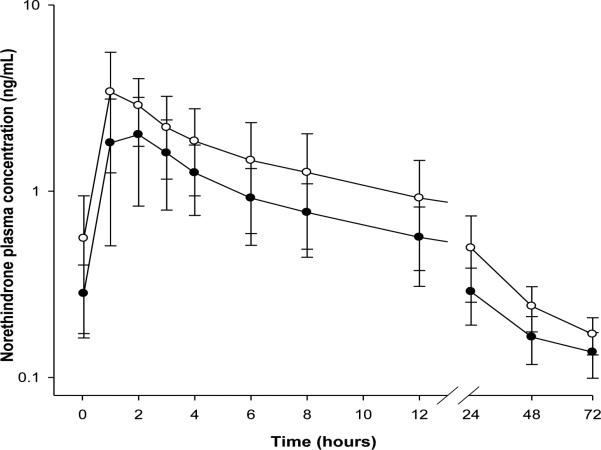
Shown in Table 2 and Figure 1 are the steady state pharmacokinetic parameters of serum norethindrone with and without ritonavir boosted atazanavir in HIV+ women. The measures of drug exposure, Cmax, C24, AUC0–24, and AUC0–∞, are significantly increased in the treatment group of women (p<0.05). An increase of 35% and 50% were observed for AUC0–∞ (33.80 (IQR: 28.28-40.93) hr*ng/mL vs. 46.10 (35.03-56.28)), and AUC0–24 (16.69 (13.28-20.55) vs. 25.20 (17.94-32.74)), respectively. Similarly, an increases of 39% and 67% were observed for Cmax (2.09 (1.49-3.06) ng/mL vs. 3.19 (2.19-4.79)), and C24 (0.27 (0.19-0.37) vs. 0.45 (0.32-0.59)),respectively. While both clearance and volume of distribution are significantly lowered in treatment group, there is no alteration in half-life (1.86 (1-2) hr vs. 1.32 (1-2)). About a 40% increase in relative bioavailability of norethindrone was observed in the treatment group of women (data not shown). There are no reports of adverse events in either group.
Table 2.
Steady state serum pharmacokinetics of oral norethindrone with and without protease inhibitor treatment in HIV+ women.
| Parameter | Control (n=17) | Treatment (n=10) | p-value |
|---|---|---|---|
| AUC0-∞ (hr*ng/mL) | 33.80 (28.28-40.93)a | 46.10 (35.03-56.28) | p = 0.037 |
| AUC0-24 (hr*ng/mL) | 16.69 (13.28-20.55) | 25.20 (17.94-32.73) | p = 0.042 |
| Vd/F (L) | 564 (432-900) | 313 (239-454) | p = 0.035 |
| CL/F (L/hr) | 10.35 (8.55-12.38) | 7.59 (6.22-10.00) | p = 0.032 |
| t1/2 (hr) | 37.78 (26.15-51.71) | 28.55 (26.23-31.89) | p = 0.167 |
| Tmax (hr) | 1.86 (1-2) | 1.32 (1-2) | p = 0.067 |
| Cmax (ng/mL) | 2.09 (1.49-3.06) | 3.19 (2.19-4.79) | p = 0.045 |
| C24 (ng/mL) | 0.27 (0.19-0.37) | 0.45 (0.32-0.59) | p = 0.010 |
data represents geometric mean (inter quartile range)
Figure 1. Norethindrone serum concentration-time profiles during steady-state.
Norethindrone serum concentrations during steady-state were estimated using radioimmunoassay in control group (closed circles) and treatment group (open circles) of women over a 72-hour period. Each data point represents mean ± SD of 17 and 10 women in control and treatment groups, respectively.
4. DISCUSSION
The current study findings parallel our earlier published findings where the treatment group included women treated with ritonavir and non-ritonavir based protease inhibitors (14). The increases in Cmax, C24, and AUCs, and corresponding relative bioavailability, were expected given inhibition of CYP3A4 by ritonavir (15). In addition to CYP3A4 inhibition, ritonavir is a known inhibitor of p-glycoprotein (18). Additionally, atazanavir is also shown to inhibit both CYP3A4 and p-glycoprotein (19). Together CYP3A4 and p-glycoprotein inhibition in gut-liver axis may have contributed to the observed increase in bioavailability of norethindrone in the treatment group of women. Therefore, unlike combined oral contraceptives, progestin only contraceptives benefit from a drug-drug interaction with ritonavir.
The increased relative bioavailability probably resulted in significant reductions in apparent clearance (CL/F) and apparent volume of distribution (Vd/F). Additionally, no change in t1/2 of norethindrone in the treatment group was observed. Given that t1/2 = Ln(2)*Vd/CL, with a proportional decrease in both Vd/F and CL/F, t1/2 would remain unchanged. An alternative explanation to changes in norethindrone pharmacokinetic parameters is offered by plasma protein binding of norethindrone, which is highly bound to both sex-hormone binding globulin and albumin (20). The decrease in Vd/F is suggestive of altered protein binding; an increase in bound fraction could result in a lower Vd/F and higher AUC. Reduction in free fraction could also offer another mechanism by which CL of a low extraction drug such as norethindrone is decreased. Currently there is no evidence in the literature suggesting protease inhibitor therapy alters serum protein concentrations. If the speculation of increased plasma protein binding theory is true, the increased total concentrations may not mean equivalent or greater efficacy if tissue penetration of norethindrone is decreased, and thus offers a potential mechanistic cue to the category-3 designation for the combination of norethindrone and ritonavir boosted protease inhibitors.
The discrepancy in serum levels of norethindrone when administered as progestin-only, compared to combination oral contraceptives, could be attributed to ethinyl estradiol. As observed by Ouellet D et al, ritonavir induces phase 2 metabolism of ethinyl estradiol leading to reduction in plasma levels (8). We speculate that reduced ethinyl estradiol levels will result in a smaller degree of induction of sex hormone binding globulin, an important plasma protein binder of norethindrone. Accordingly, reduced plasma protein binding probably elevates systemic clearance and hence overall decrease in norethindrone levels when co-administered with ethinyl estradiol (combination oral contraceptives) in HIV+ women treated with ritonavir boosted protease inhibitors. On the contrary, in progestin-only contraceptives, inhibition of CYP3A4 by ritonavir determines blood levels of norethindrone.
Potential confounders of CYP3A4 activity include viral load, body mass index, and age (16, 17). The two groups of women are similar in age, body mass index and viral load (Table 1). Higher body mass index has been associated with altered CYP enzyme activities (21), and previous work from our group demonstrates that such alterations lead to significant changes in pharmacokinetics of combined oral contraceptives (22-24). Whether obesity similarly affects pharmacokinetics of norethindrone is unclear from the current study. Doose et.al, have shown that norethindrone pharmacokinetics are unaffected by obesity, although a trend towards a ~20% decrease in both measures of drug exposure, AUC and Cmax, were noted (25). It is interesting to note that in the current study, a higher body mass index (29.2 kg/m2 vs. 25.8) was observed in control group accompanied by 30-40% lower measures of AUC and Cmax. This leads us to speculate that part of the change attributed to the treatment group may be confounded by body mass index. More importantly, our speculation reiterates the need for prospectively designed studies to tease out the relationship between obesity and norethindrone pharmacokinetics.
Based on the current study findings, we suggest that the World Health Organization recommendation about the very cautious use of oral contraceptive agents, both combination and progestin-only, is too generic a statement. Norethindrone based progestin-only contraceptives exhibit higher indices of drug exposure when co-administered with ritonavir boosted atazanavir regimen. It is logical to assume that the significant increases in pharmacokinetic exposure parameters would translate into better clinical outcomes. While this current study provides conclusive data to support the use of norethindrone based progestin-only contraception in HIV+ women using ritonavir boosted atazanavir antiretroviral therapies, we recommend caution to extrapolate these findings to other progestin-only contraceptives. Furthermore, it is unclear at this time if other combinations of protease inhibitors offer similarly favorable pharmacokinetic interactions with norethindrone based progestin-only contraception. Towards this outcome, there is a great urgency in detailed pharmacokinetic – pharmacodynamics studies of interaction between protease inhibitors and progestin-only contraceptive agents.
Implications.
Norethindrone based progestin-only contraceptives, unlike combination oral contraceptives, exhibit greater drug exposure when co-administered with ritonavir boosted atazanavir regimen, and thus may not warrant a category-3 designation by the World Health Organization. Prospective studies are needed to confirm whether pharmacokinetic interaction results in favorable clinical outcomes.
Acknowledgement of funding
This work was supported by the grant support from the office of women's health and the National Institutes of Health (Building Interdisciplinary Research Career in Women's Health - 2K12HD043488 NICHD); Society of Family Planning; Southern California Clinical and Translational Science Institute (The National Institutes of Health, National Center for Research Resources, and National Center for Advancing Translational Sciences) through Grant UL1TR000130.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential financial and other conflicting interests: None.
Clinical trial registration number: NCT01667978
REFERENCES
- 1.World Health Organization . Women and health: today's evidence tomorrow's agenda. Geneva, Switzerland: 2009. (2009) [Google Scholar]
- 2.PMTCT strategic vision 2010-2015. Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals World Health Organization. WHO Press; Switzerland: Feb 2, 2010. [Google Scholar]
- 3.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:4–9. doi: 10.1093/jac/dkh029. [DOI] [PubMed] [Google Scholar]
- 5.Korhonen T, Turpeinen M, Tolonen A, Laine K, Pelkonen O. Identification of the human cytochrome P450 enzymes involved in the in vitro biotransformation of lynestrenol and norethindrone. J Steroid Biochem Mol Biol. 2008;110:56–66. doi: 10.1016/j.jsbmb.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Department of Reproductive Health. World Health Oraganization . Medical eligibility criteria for contraceptive use. Fourth edition WHO; 2010. [Google Scholar]
- 7.Kasserra C, Li J, March B, O'Mara E. Effect of vicriviroc with or without ritonavir on oral contraceptive pharmacokinetics: a randomized, open-label, parallel-group, fixed-sequence crossover trial in healthy women. Clin Ther. 2011;33:1503–14. doi: 10.1016/j.clinthera.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–6. doi: 10.1046/j.1365-2125.1998.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:1070–8. doi: 10.1124/dmd.110.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odlind V, Weiner E, Victor A, Johansson ED. Effects on sex hormone binding globulin of different oral contraceptives containing norethisterone and lynestrenol. Br J Obstet Gynaecol. 1980;87:416–21. doi: 10.1111/j.1471-0528.1980.tb04571.x. [DOI] [PubMed] [Google Scholar]
- 11.Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–55. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 12.Broder MS, Chang EY, Bentley TG, Juday T, Uy J. Cost effectiveness of atazanavirritonavir versus lopinavir-ritonavir in treatment-naive human immunodeficiency virus-infected patients in the United States. J Med Econ. 2011;14:167–78. doi: 10.3111/13696998.2011.554932. [DOI] [PubMed] [Google Scholar]
- 13.Stanczyk FZ, Brenner PF, Mishell DR, Jr., Ortiz A, Gentzschein EK, Goebelsmann U. A radioimmunoassay for norethindrone (NET): measurement of serum NET concentrations following ingestion of NET-containing oral contraceptive steroids. Contraception. 1978;18:615–33. doi: 10.1016/0010-7824(78)90046-x. [DOI] [PubMed] [Google Scholar]
- 14.Atrio J, Stanczyk FZ, Neely M, Cherala G, Kovacs A, Mishell DR., Jr. Effect of protease inhibitors on steady-state pharmacokinetics of oral norethindrone contraception in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65:72–7. doi: 10.1097/QAI.0b013e3182a9b3f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernest CS, 2nd, Hall SD, Jones DR. Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther. 2005;312:583–91. doi: 10.1124/jpet.104.075416. [DOI] [PubMed] [Google Scholar]
- 16.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61:27–35. [PubMed] [Google Scholar]
- 17.Jones AE, Brown KC, Werner RE, Gotzkowsky K, Gaedigk A, Blake M, et al. Variability in drug metabolizing enzyme activity in HIV-infected patients. Eur J Clin Pharmacol. 2010;66:475–85. doi: 10.1007/s00228-009-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol. 1999;57:1147–52. doi: 10.1016/s0006-2952(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 19.Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–70. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- 20.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas. 2008;61:171–80. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Edelman A, Munar M, Elman MR, Koop D, Cherala G. Effect of the ethinylestradiol/levonorgestrel combined oral contraceptive on the activity of cytochrome P4503A in obese women. Br J Clin Pharmacol. 2012;74:510–4. doi: 10.1111/j.1365-2125.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–27. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelman AB, Cherala G, Munar MY, Dubois B, McInnis M, Stanczyk FZ, et al. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users. Contraception. 2013;87:220–6. doi: 10.1016/j.contraception.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelman AB, Cherala G, Munar MY, McInnis M, Stanczyk FZ, Jensen JT. Correcting oral contraceptive pharmacokinetic alterations due to obesity: a randomized controlled trial. Contraception. 2014 doi: 10.1016/j.contraception.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doose DR, Wang SS, Padmanabhan M, Schwabe S, Jacobs D, Bialer M. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540–9. doi: 10.1046/j.1528-1157.2003.55602.x. [DOI] [PubMed] [Google Scholar]