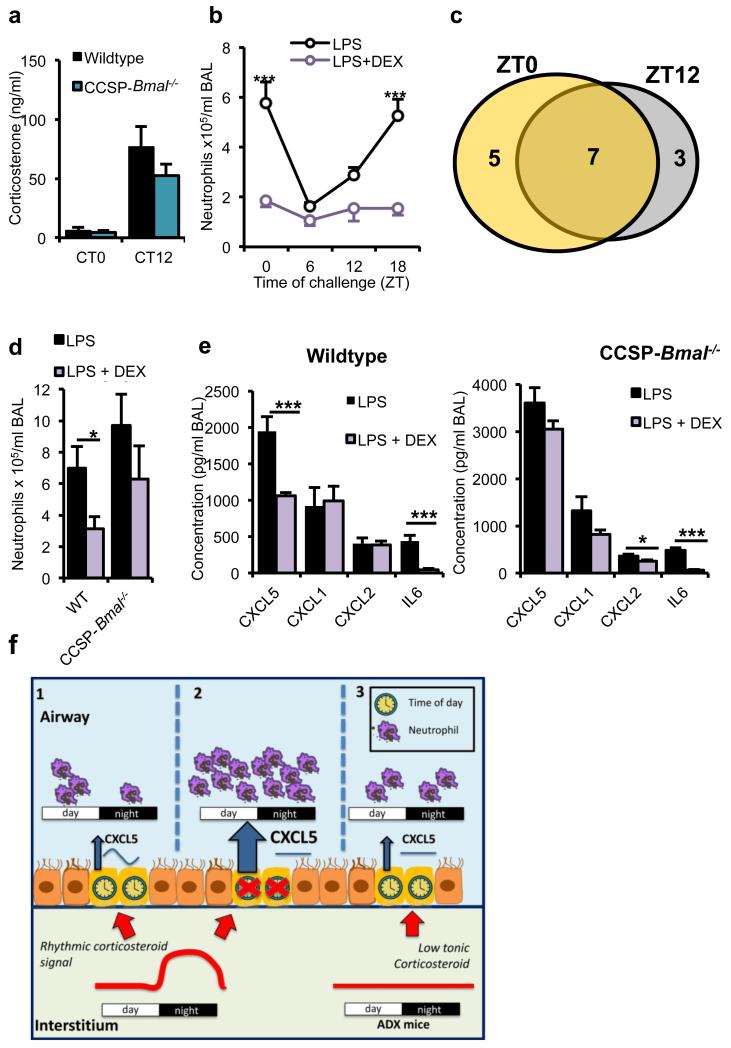
Figure 6.
Anti-inflammatory effects of glucocorticoids depend on a bronchiolar clock. a) Endogenous circadian rhythms of circulating corticosterone in CCSP-Bmal−/− mice (CT0: n = 11; CT12: n = 10) and wild-type littermates (CT0: n = 10; CT12: n = 11). (b) Effects of pretreatment with DEX (1 mg per kg body weight) on pulmonary neutrophilia in C57Bl/6 mice across the circadian day (n = 6 per time point; two-way ANOVA, post hoc Bonferonni;). (c) Venn diagram illustrating the numbers of cytokines significantly repressed by DEX at ZT0 (yellow), ZT12 (gray) or both, n = 6 per time point. (d,e) BAL neutrophil numbers after indicated treatments in CCSP-Bmal−/− (LPS: n = 6; + DEX: n = 7) and littermate (WT) controls (LPS: n = 3; + DEX: n = 6).; Student’s t-test; *P ≤ 0.05) (d) and local cytokine and chemokine production in the same study; one-way ANOVA and post hoc Bonferroni (e). (f) Schematic illustrating how a local bronchiolar clock and circulating rhythmic glucocorticoids regulate the response to timed endotoxin and bacterial challenge. In the normal state, CXCL5 is regulated by interaction of a local circadian bronchiolar clock and systemic repressive glucocorticoid signals of adrenal origin, resulting in clock-regulated responses to endotoxin and bacterial infection (1). Ablation of Bmal1 in the epithelial club cells leads to disrupted GR signaling, non-rhythmic expression of CXCL5 and neutrophilia, despite a persistent glucocorticoid rhythm (2). In this state, neutrophil chemotaxis may be impaired, leading to reduced efficiency of bacterial clearance. In adrenalectomized mice, loss of the rhythmic repressive adrenal signal leads to loss of circadian gating of CXCL5 and an increased neutrophilic response to endotoxin (3). Data are expressed as mean ± s.e.m and *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005.