Figure 2.
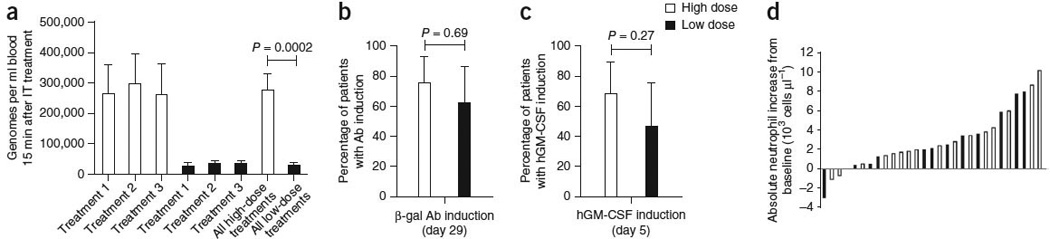
Laboratory evidence for JX-594 replication, transgene expression and GM-CSF protein function. (a) The mean (± s.e.m.) peak concentration of JX-594 (genomes measured by quantitative PCR (qPCR)) in blood after each treatment cycle (using blood obtained 15 min after the completion of treatment) by dose group (t test). IT, intratumoral. (b) The percentage of patients with evidence of β-gal transgene expression (+95% confidence interval (CI)) after JX-594 treatment (generation of antibodies (Ab) to the β-gal transgene product within 29 d of treatment is indicative of JX-594 replication, as β-gal protein expression is associated with virus replication) (Fisher’s exact test). (c) The percentage of patients with evidence of hGM-CSF transgene expression (+95% CI) on day 5 after JX-594 treatment (Fisher’s exact test). (d) Maximum induction of neutrophil concentration in blood after treatment cycle 1 by dose group (using blood obtained on days 5 and 15 after treatment). Black bars, low-dose JX-594; white bars, high-dose JX-594.
