Figure 3.
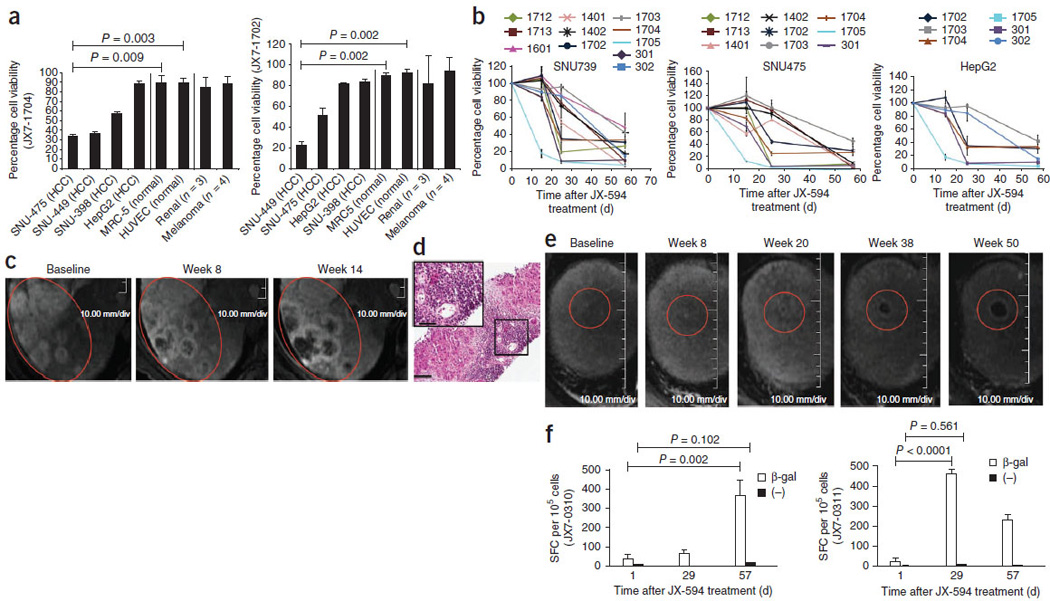
Laboratory, radiographic and biopsy evidence for JX-594– associated induction of anticancer immunity. (a) Antibody-mediated complement-dependent cytotoxicity induction after JX-594 therapy in HCC (n = 4), normal (n = 2; HUVEC and MRC-5) and non-HCC (RCC, n = 3; melanoma, n = 4) cell lines. Each graph shows the mean percentage cell viability (+s.d.) after incubation with each individual patient’s serum (diluted to 5%) collected on day 43 after the initiation of treatment compared to baseline. JX7-1704 and JX7-1702, two high-dose patients. (b) Antibody-mediated CDC induction against HCC cell lines in individual patients over time after JX-594 therapy (serum diluted to 5%; mean ± s.d.). The data shown are from serum of patients that induced ≤50% cell viability (>50% cell killing) on at least one follow-up time point. (c) Radiographic evidence of progressive necrosis and peripheral enhancement over time in noninjected tumors (JX7-0307; low dose). (d) H&E staining of a biopsy sample from a tumor collected from patient JX7-0301 (low dose) 1.5 years after the initiation of JX-594 treatment. Scale bars, 100 αm (low magnification); 50 αm (high-magnification inset). (e) Radiographic evidence of progressive necrosis and peripheral enhancement over time in a noninjected tumor (JX7-0301; low dose). Red circles (c,e) indicate the same (responding) tumors over time. (f) ELISPOT analysis detecting T cells producing interferon-γ in response to stimulation with β-gal peptides at baseline and after JX-594 treatment; data are expressed as the mean number of spot-forming cells (SFC) per 105 cells (+s.d.) (JX7-0310, low dose; JX7-0311, high dose). (−), negative control peptide. The P values in a and f were calculated by t test.
