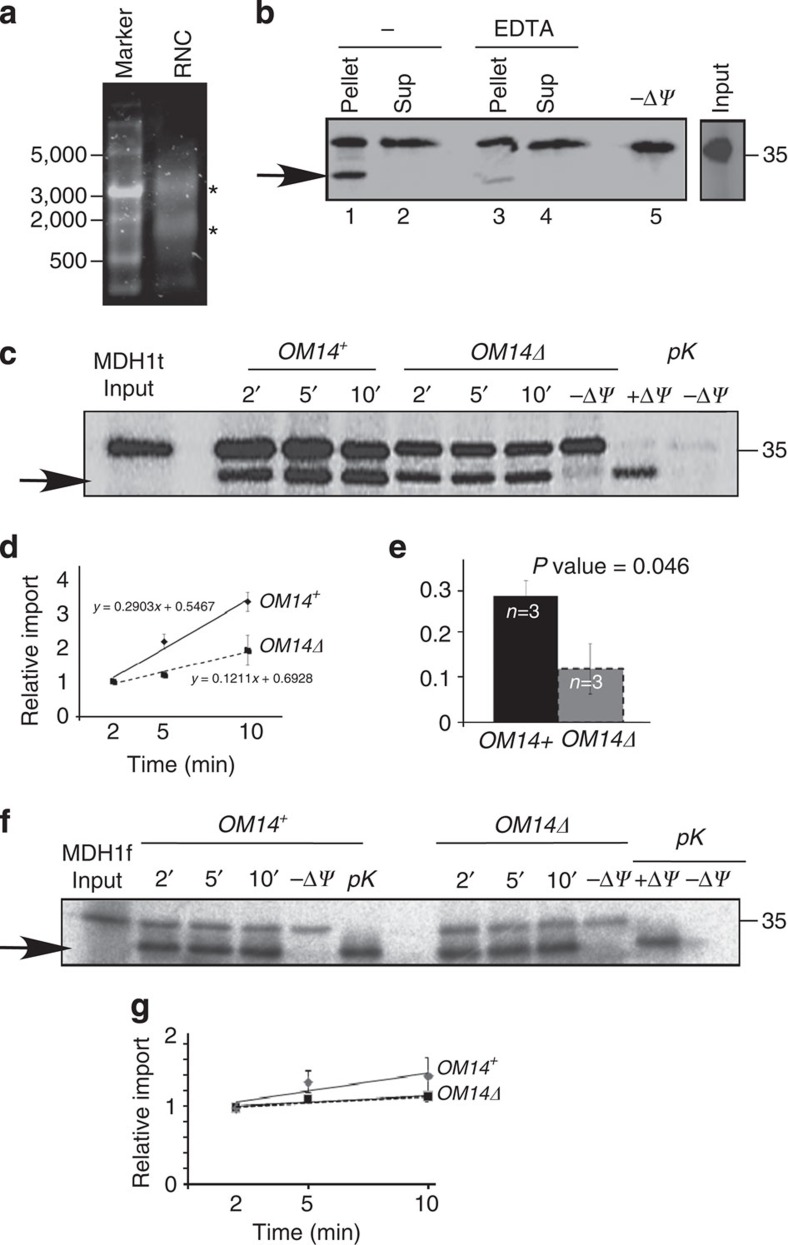
Figure 4. OM14Δ mitochondria exhibit lower co-translational import efficiency.
(a–e) Co-translational import of MDH1t precursor: (a) Stalled ribosome–nascent chains complexes were isolated by centrifugation through a sucrose cushion and mixed with highly purified mitochondria. Ethidium bromide staining of pelleted RNCs is presented. Bands at the sizes of rRNAs of the small and large subunit are indicated by asterisks. (b) RNCs labelled by 35S-Met were mixed with highly purified mitochondria (fraction five in Fig. 2b) and incubated for 5 min with or without 20 mM EDTA. Mitochondria were then isolated by centrifugation and proteins associated with mitochondria (pellet) or not (sup) were resolved on PAGE. The arrow indicates a protein that was inserted into the mitochondria and cleaved (hence it is shorter). This band is not detected in the (−ΔΨ) control reaction in which the membrane potential was diminished. Input panel is a sample from the protein labelling reaction before mixing with mitochondria. (c) MDH1t RNCs were mixed with highly purified mitochondria from OM14+ or OM14Δ cells. At the indicated time points, an aliquot was set aside and resolved on PAGE and phosphorimager. Control reactions included the addition of 1 μg ml−1 of valinomycin (−ΔΨ) during a 10 min reaction or addition of proteinase K (pK) at the end of a 10 min import reaction, to remove non-imported bands. (d) Import assays were repeated three times (n), with three time-point measurements in each. Every repeat entailed a new mitochondria prep and a new RNC prep. The average value and s.e.m. for each time point is presented. Graphs are the best-fit linear slope. (e) The histogram presents the average and s.e.m of the best-fit slopes from the three (n) independent experiments. P value was calculated by independent-samples one-sided t-test. Normal distribution was verified by standard tests (either Shapiro–Wilk or Kolmogrov–Smirnov). (f,g) Post-translational import: Full-length MDH1 (MDH1f) was synthesized from its normal ORF in a rabbit reticulocyte lysates with 35S-Met. Import assays were performed as above, with the indicated controls. Panel e presented the results of three biological repeats, with three time-point measurements in each. Every repeat entailed new mitochondria prep and a new protein synthesis reaction. The average value and s.e.m. for each time point is presented. Graphs are the best-fit linear slope. No statistically significant difference is apparent between the linear best fits of graphs (P value=0.33, independent-samples one-sided t-test).