Abstract
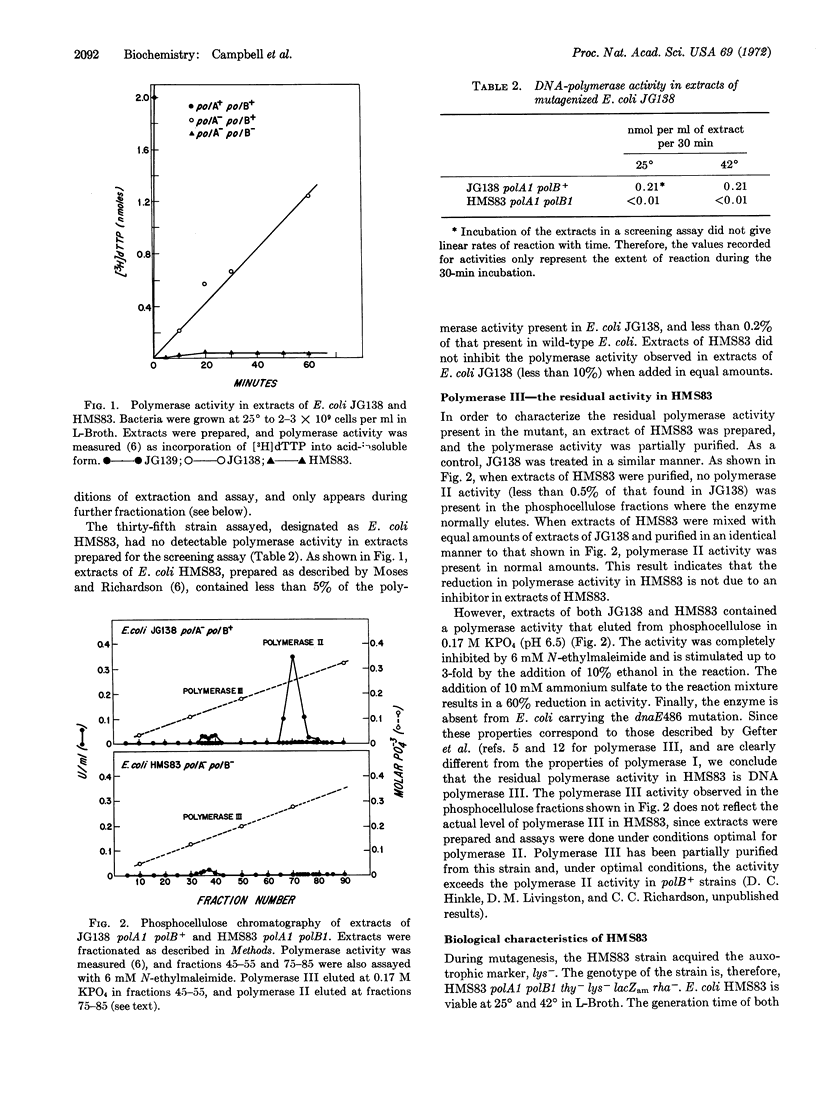
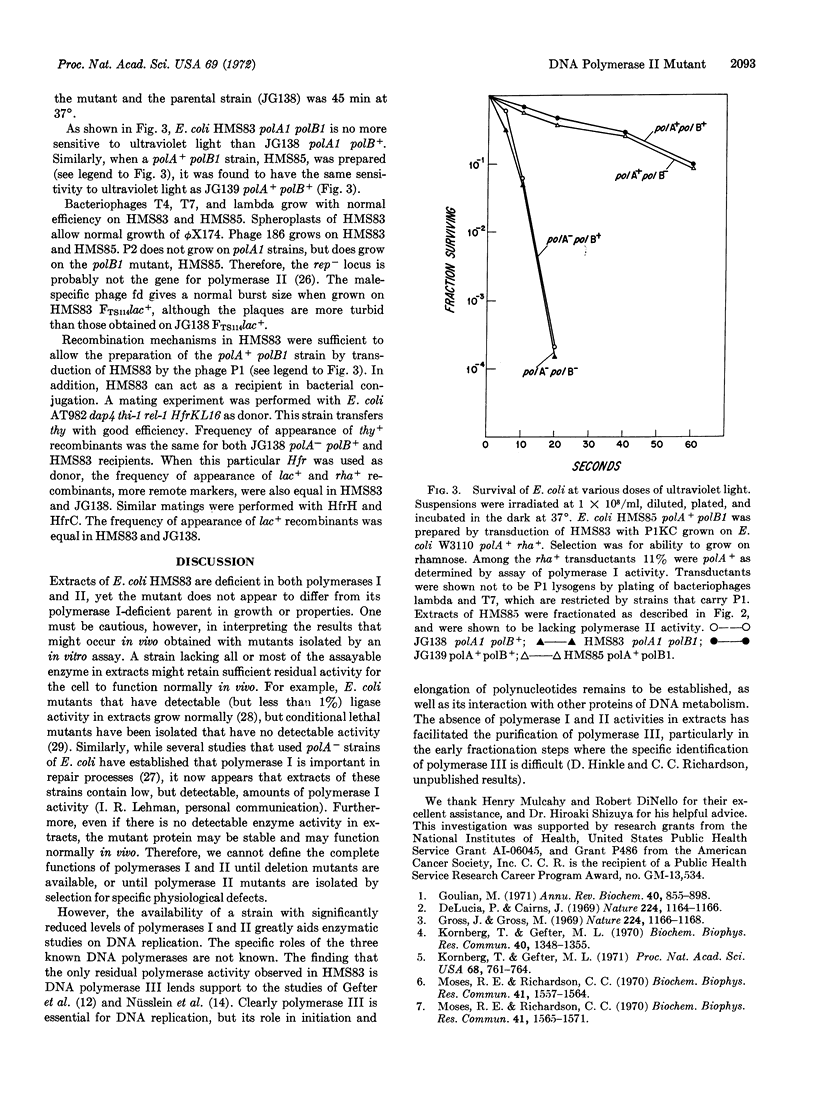
A mutant of Escherichia coli deficient in DNA polymerase II has been isolated from E. coli polA1 by mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine and assay of polymerase activity in extracts of survivors. The polA1 mutation was suppressed during mutagenesis by introduction of the suppressor, su7+, into the parental strain. The mutant, HMS83 polA1 polB1, contains less than 0.5% of the normal levels of DNA polymerase II. The only polymerase activity detected in the mutant is DNA polymerase III. E. coli HMS83 grows normally at both 25 and 42°, and supports the growth of bacteriophages T4, T7, lambda, ϕX174, 186, P2, and fd. The polB mutation does not affect sensitivity to ultraviolet irradiation or recombination frequencies.
Keywords: mutagenesis, polA1 mutants, polB1 mutants, DNA replication
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R. DNA polymerase II. Nature. 1970 Dec 12;228(5276):1050–1053. doi: 10.1038/2281050a0. [DOI] [PubMed] [Google Scholar]
- Knippers R., Strätling W. The DNA replicating capacity of isolated E. coli cell wall-membrane complexes. Nature. 1970 May 23;226(5247):713–717. doi: 10.1038/226713a0. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. DNA synthesis in cell-free extracts of a DNA polymerase-defective mutant. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1348–1355. doi: 10.1016/0006-291x(70)90014-8. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic characterization of a mutant of Escherichia coli with an altered DNA ligase. Proc Natl Acad Sci U S A. 1971 May;68(5):1002–1005. doi: 10.1073/pnas.68.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. A new DNA polymerase acitvity of Escherichia coli. II. Properties of the enzyme purified from wild-type E. coli and DNA-ts mutants. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1565–1571. doi: 10.1016/0006-291x(70)90566-8. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. A new DNA polymerase activity of Escherichia coli. I. Purification and properties of the activity present in E. coli polA1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1557–1564. doi: 10.1016/0006-291x(70)90565-6. [DOI] [PubMed] [Google Scholar]
- Nüsslein V., Otto B., Bonhoeffer F., Schaller H. Function of DNA polymerase 3 in DNA replication. Nat New Biol. 1971 Dec 29;234(52):285–286. doi: 10.1038/newbio234285a0. [DOI] [PubMed] [Google Scholar]
- Smirnov G. B., Favorskaya Y. N., Skavronskaya A. G. Monofunctional alkylating agent-induced inactivation, mutagenesis and DNA degradation in an Escherichia coli mutant deficient in DNA polymerase. Mol Gen Genet. 1971;111(4):357–367. doi: 10.1007/BF00569788. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethal nonsense suppressor in Escherichia coli which inserts glutamine. Nature. 1969 Sep 27;223(5213):1340–1342. doi: 10.1038/2231340a0. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethals: a new class of nonsense suppressors in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jun;63(2):392–399. doi: 10.1073/pnas.63.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Ginsberg B., Berkower I., Hurwitz J. Deoxyribonucleic acid plymerase II. of Escherichia coli. I. The purification and characterization of the enzyme. J Biol Chem. 1972 Jan 25;247(2):489–497. [PubMed] [Google Scholar]
- Wickner R. B., Ginsberg B., Hurwitz J. Deoxyribonucleic acid polymerase II of Escherichia coli. II. Studies of the requirements and the structure of the deoxyribonucleic acid product. J Biol Chem. 1972 Jan 25;247(2):498–504. [PubMed] [Google Scholar]



