Abstract
Objective
The surveillance of acute flaccid paralysis (AFP) is a key strategy for monitoring the progress of poliomyelitis eradication and is a sensitive measure for detecting potential cases of poliomyelitis and poliovirus infection. This study was conducted to describe the characteristics of patients reported with AFP, and to evaluate the performance of the surveillance system in Kurdistan province, western Iran, using indicators recommended by the World Health Organization (WHO).
Methods
This observational study was conducted from January 2000 to December 2010 at the Kurdistan Center for Disease Control and the Department of Pediatrics. All children who fulfilled the WHO definition for AFP were included in our study. The stool samples of all the children were sent for poliovirus isolation. All the patients were evaluated for 60 days after the onset of symptoms to identify the signs of residual weakness.
Findings
One-hundred thirty nine children aged <15 years were reported to the Center for Diseases Control with AFP. In 138 (99%) stool samples no poliovirus was isolated. None of the patients was diagnosed as having acute poliomyelitis or polio-compatible paralysis. Guillain-Barré syndrome was the most frequent final diagnosis (79 cases) followed by Transverse Myelitis (7 cases) and Encephalitis (6 cases). By detecting 1.3 to 3.6 (mean 3.2) AFP cases per 100 000 population in Kurdistan during the study period, we achieved the WHO target for AFP surveillance. All performance indicators but one consistently met the WHO requirements and therefore demonstrated the effectiveness of the AFP surveillance program in Kurdistan.
Conclusion
The effective surveillance system in Kurdistan and its evaluation may serve as a model for the surveillance of other infectious diseases.
Keywords: Poliomyelitis, Paralysis, Surveillance, Epidemiology, Acute Flaccid Paralysis
Introduction
Poliomyelitis is a highly infectious disease caused by poliovirus. It can affect any age, but primarily involves children aged less than 5 years and causes paralysis in one out of every 200 to 1000 infected individuals[1, 2]. Eradication of poliomyelitis is attained through widespread immunization, and vigilant surveillance for acute flaccid paralysis (AFP), reinforced with laboratory implementation[3, 4]. Surveillance should be performed not only for paralytic poliomyelitis, but also for other conditions including Guillain-Barré syndrome (GBS), among children aged less than 15 years. Therefore, the surveillance system was organized to identify the clinically suspected cases of poliomyelitis, and then to track them using laboratory investigations to either rule in or rule out the diagnosis of poliomyelitis caused by the wild poliovirus.
AFP surveillance is a key strategy for monitoring the progress of polio eradication and is a sensitive measure for detecting the potential cases of poliomyelitis and poliovirus infection. The World Health Organization (WHO) has devised a set of performance indicators to ensure that AFP surveillance is properly maintained[1, 5, 6]. Evaluation of AFP surveillance should be based on these performance indicators.
The Polio Eradication Program was started in Iran in 1991. In 1998, an active surveillance program for AFP was established by the Iranian Government based on new indictors recommended by WHO guidelines[7]. Kurdistan is one of the largest provinces in west Iran, with a population of about 1.5 million[8, 9]. The last case of poliomyelitis was reported in 1995. Kurdistan has the largest common border with Iraq and may be the portal tray of poliomyelitis from this country. On the other hand, Afghanistan and Pakistan represent a common epidemiological reservoir of poliovirus. The indigenous transmission of wild poliovirus in these countries has never been eradicated. The long border of Iran with these two countries requires continuous and vigilant surveillance system to timely detect poliomyelitis cases. Poliomyelitis has been a statutory reportable infectious disease in Kurdistan.
We aimed to describe the characteristics of patients reported with AFP, and to evaluate the performance of the AFP surveillance system using indicators recommended by the WHO. Our study summarizes the findings of the AFP surveillance conducted in Kurdistan during 2000-2010.
Subjects and Methods
This observational study was conducted at the Kurdistan Center for Diseases Control and the Department of Pediatrics, Kurdistan University of Medical Sciences. The study was done during January 2000 to December 2010.
The four steps of AFP surveillance recommended by WHO were followed as[9, 10]:
Finding and reporting children with AFP
Transporting stool samples for analysis
Isolating and identifying poliovirus in the laboratory
Mapping the virus to determine the origin of the virus strain.
Initially, a questionnaire was designed, based on data recommended by WHO guidelines[5]. Case definition (of what should be reported) was embedded and defined clearly. The WHO has defined AFP as “any child under 15 years of age with acute onset of focal weakness or paralysis (including GBS)” or any person with paralytic illness at any age when polio suspected, characterized by flaccid (reduced tone), without other obvious causes (e.g. trauma)[5, 9]. Transient weaknesses such as postictal weakness should not be reported as AFP[11]. Data regarding the demographic characteristics of the patients, dates of evaluation, diagnosis, follow-up, and laboratory findings were included in the questionnaire. All documents including copies of the patients’ charts, laboratory findings, imaging and electrodiagnostic evaluations (electromyography [EMG] and nerve conduction velocity studies [NCS]) even if performed in other hospitals were collected in an exclusive file.
Data were sought out by visiting or contacting a feed-forward site. Medical records and registers of the site were reviewed to identify the cases.
The staffs in all health facilities – from district health centers to large hospitals were educated to promptly report every case of acute flaccid paralysis (AFP) in any child less than 15 years of age. Our public health staff made regular weekly visits to hospitals and all private medical clinics to search for AFP cases which may have been overlooked or misdiagnosed. A specially designed form had to be signed by attending physicians in the health care centers for any suspicious case or "zero reporting" of AFP. All children with AFP were reported and tested for wild poliovirus within 48 hours of onset, even if the physicians were confident on clinical grounds that the child did not have polio. Close contacts of the patients were traced to ensure that they had been fully immunized.
According to the WHO guidelines, which require that ‘adequate’ stool samples be collected within 14 days of the onset of paralysis, two specimens – taken 24–48 hours apart – were gathered. Stool specimens were sealed in containers and stored immediately inside a refrigerator or packed between frozen ice packs at 4–8°C in a cold box, and shipped to the national laboratory in Tehran within 72 hours of collection. The National Polio Laboratory (NLP) is located at the School of Public Health affiliated to Tehran University of Medical Sciences and has been integrated into the network of polio laboratories run by the World Health Organization since 1996. It is also a WHO-accredited laboratory to perform intra-typic differentiation of polioviruses[12]. More recently, the Iran National Polio intratypic differentiation (ITD) Laboratory has been accredited again by WHO from May 2010 to April 2011 based on the overall review for 12 months. The Laboratory had passed the proficiency test.
From January 2000 to December 2010, all children who fulfilled the WHO definition for AFP were included in our study. All the patients were evaluated for 60 days after the onset of symptoms to identify the signs of residual weakness. The AFP committee of the Kurdistan University of Medical Sciences formed an Expert Panel consisting of three consultants including a pediatric infectious diseases sub-specialist, a neurologist, and an infectious diseases specialist to classify the AFP cases according to the WHO recommendations [5, 6, 9]. The WHO protocol for case classification was followed to determine which patients should undergo review by the expert panel (Fig. 1). This group included patients whose stool specimens were insufficient for laboratory diagnosis, who still suffered from residual weakness, or who had not been followed up. A detailed case report was given to each panel member who independently evaluated each case. The panel members’ views and comments were documented and a consensus was reached for the final classification of each patient. The diagnosis was made following a complete review of the clinical and epidemiological data, EMG and NCS studies follow up outcome and laboratory results of stool cultures for polio and non-polio enteroviruses provided by NPL. The AFP committee designed a special sheet and feed forward forms. These forms were filled up and signed by members of the provincial committee every 3 months and faxed to the National Expert Committee (NEC) in Tehran. If the cause of a case of AFP could not be determined at the provincial level, the case was referred to the NEC, which was responsible for the final diagnosis. This committee consists of eight experts (a pediatrician, virologist, infectious diseases specialist, neurologist, and an epidemiologist). NCC convenes several times per year, reviews reports, interacts with the provincial committee and the NLP, and prepares an annual report of the situation for the WHO Eastern Mediterranean Regional Office (EMRO)[13]. After final approval of the data by NCC, feedback was provided to the provincial committee.
Fig. 1.
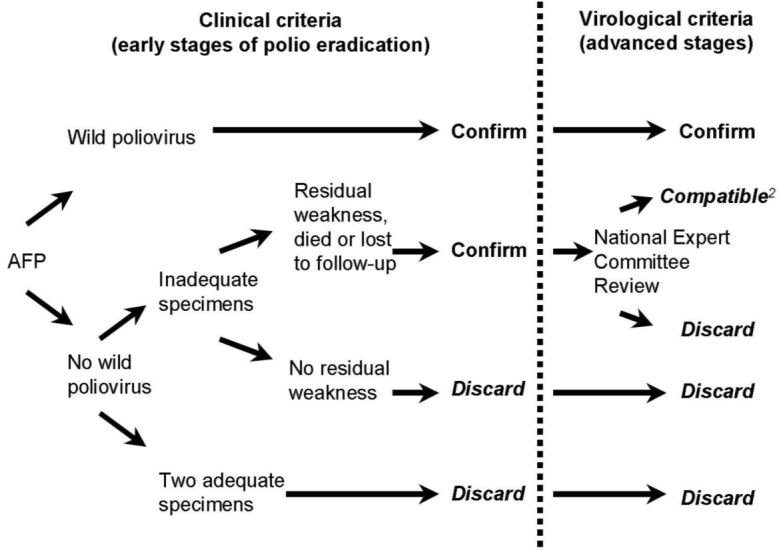
Virological flowchart and case classifications.[6, 9] (The permission citing this figure was obtained from the WHO, with the permission ID: 102317)
The detected cases were divided into four different age groups. Then in order to estimate the annual incidence rate of AFP in population aged less than 15, we used the Poisson regression. The data were analyzed using R software, version 2.15 to generate statistics based on the WHO performance indicators. P-values below 0.05 were considered statistically significant.
Findings
One-hundred thirty nine children with acute flaccid paralysis aged from 2 months to 14 years were reported to the Center for Diseases Control during 2000–2010 periods. Table 1 shows the incidence of AFP by age and sex. According to the Poisson regression analysis, the incidence of diseases manifesting as AFP such as GBS were highest in early years of age, 66% of patients were less than 6 years old [Relative Risk (RR) =5.93, 95% Confidence interval (CI): 3.2, 10.7, P<0.001]. Males comprised a slightly higher percentage (55%) of cases than females. However this difference was not statistically significant [RR=0.93, 95% CI: 0.77, 1.12, (P=0.5)]. Sixty percent of children were from urban areas.
Table 1.
Mean annual incidence rate of AFP per 100,000 under 15 year old children by age and gender using Poisson regression analysis
| Variable | - | Number (%) | Population | AFP Rate | Relative risk | 95% CI | P. value 1 |
|---|---|---|---|---|---|---|---|
| Age groups (years) | 0-1 | 34 (24.5) | 44991 | 6.8 | 5.93 | (3.27-10.78) | <0.001 |
| 2-5 | 58 (41.7) | 87330 | 6 | 5.48 | (3.15-9.52) | <0.001 | |
| 6-10 | 31 (22.3) | 113963 | 2.4 | 2.20 | (1.21-4.03) | 0.01 | |
| 11-14 | 16 (11.5) | 131768 | 1.1 | 1 | - | - | |
| Gender | Female | 63 (45.3) | 183994 | 3.1 | 1 | - | - |
| Male | 76 (54.6) | 194058 | 3.5 | 1.15 | (0.82-1.6) | 0.4 | |
| Sum | 139 | 378052 | 3.34 | - | - | - |
AFP: acute flaccid paralysis; CI: Confidence interval
In 139 (100%) of the cases stool samples were sent for poliovirus isolation. Stool examination was adequate in all cases and was sufficient to exclude poliomyelitis. In 138 (99%) stool samples no poliovirus was isolated (Table 2). In one stool sample, vaccine poliovirus was isolated. This child was vaccinated one week after the occurrence of paralysis and therefore paralysis was not related to polio vaccination. The child had an uneventful recovery.
Table 2.
AFP Surveillance Indicator Statistics in Kurdistan province comparing with Iran and EMRO in total
| Kurdistan | Iran | EMRO | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO Indicator | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Mean 2000-2010 | 2009-2010 | 2009 | 2010 | |
| Population Size (<15 yrs) | 500200 | 476857 | 451361 | 437335 | 417574 | 398782 | 378052 | 358073 | 337661 | 378052 | 391035 | 407210 | 18713773a | 242131455a | ||
| Number of AFP cases | 12 | 12 | 6 | 11 | 9 | 8 | 23 | 17 | 19 | 8 | 14 | 12.6 | 549 | 622 | 10611 | 11338 |
| AFP incidence | 2.4 | 2.5 | 1.3 | 2.5 | 2.15 | 2 | 6.1 | 4.7 | 5.6 | 2.1 | 3.6 | 3.2 | 3.1 | 3.3 | 4.4 | 5.6 |
| Number/Incidence of Guillain-Barré | 9/1.8 | 10/2.1 | 4/0.9 | 6/1.37 | 7.1.68 | 4.1 | 9/2.4 | 9/2.5 | 12/3.5 | 5/1.5 | 4/1 | 7.2.1.7 | -b | -b | -b | -b |
| Stool adequacy 80%< | 93% | 92% | 86% | 91% | 100% | 90% | 92% | 100% | 100% | 100% | 100% | 95% | 84% | 91% | 91% | 91% |
| OPV3 coverage rates c | - | - | - | - | 100% | 99.8% | - | - | 100% | 100% | 100% | - | 97.1% | 98% | 82% | 85% |
| NPEV isolation rate (10%) | 7.1% | 15.4% | 14.3% | 9.1% | 0 | 0 | 4.2% | 5.9% | 0 | 0 | 0 | 5% | 3% | 2% | 15% | 17% |
| Time between collection of first stool and receipt in the NPL (80%) | 93% | 92% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 98% | 100% | 100% | 94% | 94% |
| Follow-up for residual paralysis at 60 days | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 97% | 96% |
EMRO, Eastern Mediterranean Regional Office, AFP, acute flaccid paralysis, OPV3, 3 doses of oral polio vaccine, NPEV, non-polio enterovirus, NPL, National polio laboratory
a: Population (<15 yrs) in 2010
b: The mean reported Incidence of Guillain-Barré syndrome for Iran is 2.8 and for USA and globally is 1.5 [12–20].
c: The data are retrieved from 5 studies based on cluster sampling conducted in Kurdistan Center for Diseases Control.
During the study period, all cases were classified according to the virological flowchart (Fig. 1). The Expert Panel reviewed 139 cases. Diagnoses of the 139 confirmed cases are shown in Table 3. The major clinical causes of AFP were GBS (56.8%), central nervous system (CNS) infection (4.3%), CNS tumors (5.1%), transverse myelitis (5%), myasthenia gravis (2 cases), hypokalemic paralysis (2 cases), and botulism (one case). The presentations of 3 cases of GBS were as Miller Fischer syndrome. The diagnosis of GBS was based on the classic clinical feature of symmetric ascending paralysis. In the 37% of the cases EMG and NCS were performed but cerebrospinal fluid analysis data were available for only 13% of the cases. In some cases with dominant CNS manifestation, MRI or CT scan of the brain or spinal cord was performed to rule out mass occupying lesions. Most children had an uneventful recovery, but residual weakness persisted in 28 (20%) patients. Four children with the final diagnosis of GBS (1 case), encephalitis (1 case), congenital myopathy (1 case) and metabolic encephalopathy (1 case) expired. None of the children was classified as polio-compatible or having acute poliomyelitis. Eleven cases that were primarily reported as AFP by primary care physicians were discarded by the AFP provincial committee because their clinical pictures were not compatible with the definition of acute flaccid paralysis[14]. The stool cultures for all of them were negative and all had an uneventful recovery. The results of these cases were not counted in the final report to avoid over diagnosis and over-counting the AFP cases. All of these cases with their classification were reported to NEC and feedback was provided by NEC.
Table 3.
Number of Cases (%) with neurological diagnoses of acute flaccid paralysis (AFP) from 2000 to 2010 comparing to other reports
| Our Study | Davarpanah et al[15] | Poorolajal et al[28] | Desai S et ala CPSPb)[29–32] | IHMI Hussain et al[19] | D’Souza et al[17] | ||
|---|---|---|---|---|---|---|---|
| Location (District/Country) | Kurdistan/Iran | Shiraz/Iran | Hamadan/Iran | Canada | Malaysia | Australia | |
| Diagnoses | Time Period | 2000-2010 | 1995-2006 | 2002 to 2009 | 1996-2010 | 1997-2001 | 1995-1998 |
| Guillain-Barré syndrome | 79 (56.8) | 150 (66) | 66 (75) | 485 (73.8) | 156 (30.2) | 52 (46.9) | |
| Central nervous system infection | 6 (5) | 4 (2) | - | 10 (1.5) | 84 (16.2) | 7 (6.3) | |
| Transverse myelitis | 7 (5) | 3 (1) | 2 (2.2) | 77 (11.7) | 55 (10.6) | 21 (18.9) | |
| Traumatic neuritis | 3 (2.2) | 3(1) | 1 (1) | 1 (0.15) | 8 (1.5) | - | |
| Myositis (viral) | 4 (2.9) | - | - | 1 (0.15) | 13 (2.5) | 1 (0.9) | |
| Central nervous system tumor | 3 (2.2) | 6 (3) | 1 (1) | - | - | 1 (0.9) | |
| Hypokalemic periodic paralysis | 2 (1.4) | - | - | - | 27 (5.2) | - | |
| Spinal tumors/lesions | 4(2.9) | - | - | 2 (0.3) | 7 (1.4) | 8 (7.2) | |
| Acute cerebellar ataxia | 3 (2.2) | - | - | 1 (0.15) | 5 (1.0) | - | |
| Toxic synovitis of hip | 6 (4.3) | - | - | - | - | - | |
| Myasthenia gravis | 2 (1.4) | - | - | 1 (0.15) | - | 2 (1.8) | |
| Botulism | 1 (0.7) | - | - | 3 (0.4) | 3 (0.6) | 1 (0.9) | |
| Other diagnoses a | 19 (13)c | 60 (26) | 4 (4.5) | 70 (10.6) | 45 (8.7) | 15 (13.5) | |
| Total AFP reported | 150 | 227 | 88 | 1040 | 517 | 171 | |
| Unclassified | - | - | 14 (16) | 6 (0.9%) | 62 (12.0) | 2 (1.8) | |
| Discarded/not met the AFP case definition | 11d (7.3) e | - | - | 383 (37) e | - | 60 (35) e | |
| Total AFP confirmed | 139 | 227 | 74 | 657 | 517 | 111 | |
| Polio compatible | - | 1 (0.9) | - | - | 3 (0.6) | 1 (0.9) | |
a: Data modified from yearly reports of Canadian Pediatric Surveillance Program 1996-2010. Available at: http://www.cps.ca/English/surveillance/cpsp/studies/acute.htm
b: Canadian Pediatric Surveillance Program
c: Other diagnoses were intracranial hemorrhage(one case), ischemic myelopathy (one case), cranial palsy (one case), occult spinal trauma (one case), epilepsy (one case), congenital myopathy (one case), metabolic encephalopathy (one case), congenital hypomyelinating neuropathy (one case), leukoencephalopathy (one case), neurodegenerative disease (one case), hypoxic ischemic encephalopathy (one case)
d: Eleven cases that were primarily reported as AFP by primary care physicians were discarded by the AFP provincial committee because their clinical pictures were not compatible with the definition of acute flaccid paralysis.
e: Percent of Discarded AFP cases/total AFP cases
The overall incidence of AFP was calculated as 1.3 to 3.6 (mean 3.2) per 100 000 population in Kurdistan during the study period (Table 2). The incidence was lowest in the early years of the study and received highest (6.1) in 2006. The mean incidence of GBS was calculated as 1.7.
During the study, a high coverage rate for poliovirus vaccination was maintained. Based on several studies with cluster sampling, the coverage rate for 3 doses of oral poliovirus vaccine remained well high at about 100% (Table 2).
The peak incidences of AFP occurred during May (14 patients) and June (17 patients), which coincides with the higher enterovirus activity associated with summer months (Fig. 2).
Fig. 2.
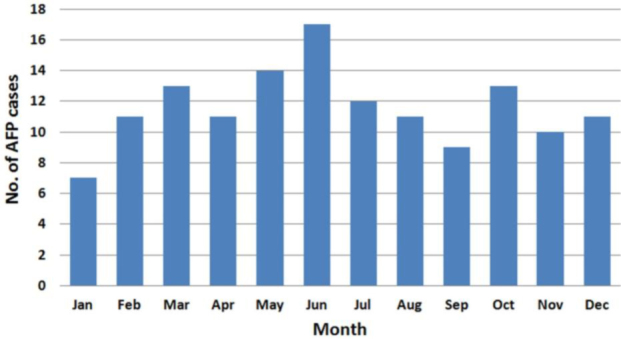
No. of acute flaccid paralysis (AFP) cases reported by month from 2000 to 2010
Discussion
GBS was the most common cause of AFP in our study, which is consistent with the findings of other studies[15–19]. This syndrome occurs throughout the world with an annual incidence of 0.4–4 (Mean 1.3) cases per 100 000 population[20–27]. Our surveillance data suggest that about seven cases of GBS per year will be diagnosed with paralytic presentations in Kurdistan. Considering the population in Kurdistan, this figure for individuals under 15 years of age gives a mean incidence of 1.7 per 100000.
The diversity of end diagnosis of AFP patients was similar to other reports with some differences (Table 3)[15,17–19,28–32]. Transverse myelitis and encephalitis were the second and third diagnosis in number. Myositis and toxic synovitis of the hip were among the less frequent diagnoses in other series[17, 19, 33, 34]. Notably, in a few reports trauma or posttraumatic lesions had been reported as AFP[17]. In some cases, the underlying mechanism initially had been obscure so necessitated investigation of the cases to rule out poliomyelitis. However when the trauma is clear mechanism of the paralysis, it should not be reported as AFP in the final report[5, 9].
The diagnoses of AFP is very diverse because many diseases can mimic symptoms and signs of poliomyelitis[9]. The AFP surveillance system is a syndromic approach to diagnosis of poliomyelitis. Accordingly, the diagnosis reports should be mostly based on symptoms and signs of a specific involved organ system. Etiological diagnosis should be reserved for the specific diagnosis of poliomyelitis. Using both organ specific and etiological agents for reporting diagnoses other than poliomyelitis may be misleading in developing countries. In many developing countries, diagnosis by etiological agent has only been possible in remote specialized reference laboratories for poliovirus using specific culture media. The AFP surveillance system should be based on a unique guideline for reporting specific diagnoses to be able to compare results of many locations and countries. This improves the legal framework for coordinating efforts to confine poliomyelitis.
The WHO has defined the target of detecting at least one AFP case per 100 000 in children under15 years of age per year as a measure to evaluate the effectiveness of AFP surveillance[4]. This ratio was increased to two cases per 100 000 because Iran is near polio-endemic countries such as Afghanistan, Pakistan and Iraq[35]. By detecting 1.3 to 3.6 (mean 3.2) cases per 100 000 population in Kurdistan comparing 3.1 to 3.3 rate for Iran and 4.4 to 5.6 for Eastern Mediterranean Region of WHO during the study period, we achieved the WHO target for AFP surveillance.
The number of reported cases of AFP was relatively higher during the summer months because of higher enterovirus activity. However, the AFP incidence is mostly a reflection of GBS incidence. Data about prevalence of GBS in special seasons are inconsistent[36, 37]. Most studies indicate that this syndrome is sporadic without significant variation over time[23, 24]. Adequate virological examination was performed for more than 90% of patients (Table 2). Virus isolation from feces or throat swabs was successful for only a minimum of 5% of the patients. WHO target for non-polio enterovirus isolation rate is 10%[5]. This indicator remained regularly below the acceptable rate in recent years in our country (Table 2). According to Polio Laboratory Network update "A major change in 2000 is the substitution of poliovirus-specific L20B cells for NPEV-sensitive Hep2 cells in National Reference Polio Laboratories. Because isolation of NPEV now depends solely on RD cells, annual rates for most laboratories are expected to be lower than in previous years." How much lower is still unclear[38]. The NPEV rate is no longer a strict criterion for accreditation of reference laboratories because the isolation rate is affected by various factors such as the season of the year, altitude, or population hygiene levels[39]. However, the rate may be a useful indicator of laboratory performance. Consequently, there is a need to improve the virological culture technique of patients with AFP in our country. Other performance indicators have met WHO criteria with regard to AFP rate, timeliness of reporting, and adequate epidemiological investigations (Table 2). These indicators demonstrated the effectiveness of the AFP surveillance program in Kurdistan.
Our report shows that improvements are needed, particularly with regard to laboratory investigation of AFP cases. "Satisfactory AFP surveillance programs rely on three factors: detection, investigation, and timely reporting of cases[1]." AFP surveillance results in our region indicate that vigilance in case detection and reporting meet the WHO standards. Meanwhile, the increase in notifications suggests an increased rate of enthusiasm in reporting. This seems to be influenced by an increase in the physicians’ awareness. The completeness and accuracy of reporting was further improved by reviewing hospital discharge records as secondary data sources.
Our study was disadvantageous for the some disorganization of data collected during early years of the study. Another limitation of our study is that the data collection using questionnaires was done by health care workers and therefore, some answers could be subject to error and may be less reliable.
Conclusion
The surveillance system for AFP was effective in Kurdistan province and compares well in most aspects with other systems in the Eastern Mediterranean region. The surveillance system and its evaluation may serve as a model for surveillance of other infectious diseases.
Acknowledgment
The authors would like to acknowledge the valuable contributions of Dr. Farzam Bidarpoor, vice chancellors of health of Kurdistan University of Medical Sciences whose participation made this study possible. Kurdistan University of Medical Sciences supported the whole study.
Authors’ Contribution
Conception and design, acquisition of data, analysis and interpretation of data: J. Soltani, N. Esmailnasab, D. Roshani
Acquisition of data, patient selection and follow up: M Karimi, M.J. Amjadi.
All Authors participated in drafting the article and approved the final manuscript as submitted.
Conflict of Interest
None
References
- Lam RM, Tsang TH, Chan KY, et al. Surveillance of acute flaccid paralysis in Hong Kong: 1997 to 2002. Hong Kong Med J 2005;11(3):164–73. [PubMed] [Google Scholar]
- Reynolds T.Polio: an end in sight? BMJ 2007; 335(7625):852–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Polio Eradication Initiative., World Health Organization. Dept. of Vaccines and Biologicals.Global Polio Eradication Initiative: strategic plan 2004-2008. Geneva: World Health Organization.2003. [Google Scholar]
- World Health Organization. Dept. of Vaccines and Biologicals.Global Polio Eradication Initiative : strategic plan 2001–2005. Geneva: World Health Organization.2000. [Google Scholar]
- World Health Organization. Dept. of Vaccines and Biologicals.WHO-recommended standards for surveillance of selected vaccine preventable diseases. Geneva: World Health Organization.2003. [Google Scholar]
- Acute flaccid paralysis surveillance: the surveillance strategy for poliomyelitis eradication. Weekly Epidemiol Record 1998;73(19):113-7. [Google Scholar]
- Zahraei S, Sadrizadeh B, Gouya M.Eradication of poliomyelitis in Iran, a historical perspective. Iran J Public Health 2009;38(Suppl. 1):124–6. [Google Scholar]
- Population by Type of Household, Sex and Ostan, 1385 Census. Statistical Center of Iran. Available at: http://amar.sci.org.ir/Detail.aspx?Ln=E&no=98493&S=SS; accessed March28, 2013.
- World Health Organization. Dept. of Vaccines and Biologicals. Logistics management.Geneva: World Health Organization; 2001. [Google Scholar]
- World Health Organization. Regional Office for Europe.Review of documentation for certification of poliomyelitis eradication (Denmark, Finland, Netherlands, United Kingdom): report on the fifth certification commission meeting, Copenhagen, Denmark 27–29 April 1998. Copenhagen: WHO Regional Office for Europe.1998. [Google Scholar]
- Buck P, Herman S, Scott C, et al. Protocol for the investigation of acute flaccid paralysis and suspected paralytic poliomyelitis. Can Commun Dis Rep 1998; 24(4):25–32. [PubMed] [Google Scholar]
- Polio Eradication Initiative, Country activity, Islamic Republic of Iran. Cairo, Egypt: official Website of Eastern Mediterranean Regional Office, World Health Organization. Available at: http://www.emro.who.int/polio/countries/islamic-republic-of-iran.html.accessed July20, 2012.
- Taha Moussavi M, Bijan Sadrizadeh M, Mohsen Zahraei M, et al. Polio Eradication in Iran. Arch Iran Med 2012; 15(2):107–9. [PubMed] [Google Scholar]
- Marx A, Glass JD, Sutter RW.Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiol Rev 2000;22(2):298–316. [DOI] [PubMed] [Google Scholar]
- Davarpanah M, Bakhtiari H, Mehrabani D, et al. A 12-years surveillance of poliomyelitis and acute flaccid paralysis in Fars Province, Southern Iran. Iran Red Crescent Med J 2008; 10(4):288–93. [Google Scholar]
- Whitfield K, Kelly H.Using the two-source capture-recapture method to estimate the incidence of acute flaccid paralysis in Victoria, Australia. Bull World Health Organ 2002; 80(11):846–51. [PMC free article] [PubMed] [Google Scholar]
- D’Souza R, Kennett M, Antony J, et al. Acute flaccid paralysis surveillance in Australia progress report 1995-1998. Commun Dis Intell 1999; 23(5):128–31. [PubMed] [Google Scholar]
- Hussain IH, Ali S, Sinniah M, et al. Five-year surveillance of acute flaccid paralysis in Malaysia. J Paediatr Child Health 2004; 40(3):127–30. [DOI] [PubMed] [Google Scholar]
- Varughese P.Acute flaccid paralysis, Canadian Paediatric Surveillance Program - 2003 Results. Canadian Paediatric Society (Public Health Agency of Canada). Available at: http://www.phac-aspc.gc.ca/publicat/cpsp-pcsp03/page4-eng.php.accessed June10, 2012.
- Kuwabara S.Guillain-Barre syndrome: epidemio-logy, pathophysiology and management. Drugs 2004; 64(6):597-610. [DOI] [PubMed] [Google Scholar]
- Molinero MR, Varon D, Holden KR, et al. Epidemiology of childhood Guillain-Barré Syndrome as a cause of acute flaccid paralysis in Honduras: 1989-1999. J child Neurol 2003; 18(11):741–7. [DOI] [PubMed] [Google Scholar]
- Barzegar M, Alizadeh A, Toopchizadeh V, et al. Association of Campylobacter jejuni infection and GuillainBarre syndrome: a cohort study in the northwest of Iran. Turk J Pediatr 2008; 50(5):443–8. [PubMed] [Google Scholar]
- Hughes RA, Rees JH.Clinical and epidemiologic features of Guillain-Barre syndrome. J Infect Dis 1997; 176 Suppl 2:S92–8. [DOI] [PubMed] [Google Scholar]
- McGrogan A, Madle GC, Seaman HE, et al. The epidemiology of Guillain-Barre syndrome worldwide. A systematic literature review. Neuroepidemiology 2009;32(2):150–63. [DOI] [PubMed] [Google Scholar]
- Prevots DR, Sutter RW.Assessment of Guillain-Barré syndrome mortality and morbidity in the United States: implications for acute flaccid paralysis surveillance. J Infec Dis 1997; 175(Suppl 1):S151. [DOI] [PubMed] [Google Scholar]
- Arami M, Yazdchi M, Khandaghi R.Epidemiology and characteristics of Guillain-Barre syndrome in the northwest of Iran. Ann Saudi Med 2006; 26(1):22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteghamati A, Gouya MM, Keshtkar AA, et al. Relationship between occurrence of Guillain-Barre syndrome and mass campaign of measles and rubella immunization in Iranian 5–14 years old children. Vaccine 2008; 26(39):5058–61. [DOI] [PubMed] [Google Scholar]
- Poorolajal J, Ghasemi S, Farahani LN, et al. Evaluation of acute flaccid paralysis in Hamadan, Iran from 2002 to 2009. Epidemiol Health 2011; 33: e2011011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey J, Lipskie T.Acute flaccid paralysis, Canadian Paediatric Surveillance Program - 2007 Results; Available at: http://www.cpsp.cps.ca/uploads/publications/Results-2007.pdf.2007, Pp:19–22, accessed July29, 2012. [Google Scholar]
- Desai S, Garner M.Acute flaccid paralysis, Canadian Paediatric Surveillance Program - 2008 Results; Available at: http://www.cpsp.cps.ca/uploads/publications/Results-2008.pdf.2008, Pp:33–36, accessed July29, 2012. [Google Scholar]
- Desai S, Helferty M.Acute flaccid paralysis, Canadian Paediatric Surveillance Program - 2009 Results; Available at: http://www.cpsp.cps.ca/uploads/publications/Results-2009.pdf.2009, Pp:16–21, accessed July29, 2012. [Google Scholar]
- Desai S.Acute flaccid paralysis, Canadian Paediatric Surveillance Program - 2010 Results, Available at: http://www.cpsp.cps.ca/uploads/publications/Results-2010.pdf.2010, Pp:15–18, accessed July29, 2012. [Google Scholar]
- Oostvogel P, Spaendonck M, Hirasing R, et al. Surveillance of acute flaccid paralysis in The Netherlands, 1992–1994 Bull World Health Organ 1998; 76(1): 55. [PMC free article] [PubMed] [Google Scholar]
- Tal-hatu KH, Omotade TT.Acute flaccid paralysis: a five–year review of cases managed by physiotherapy at the University College Hospital, Ibadan. Afr J Health Sci 2006;13(1-2):28–32. [DOI] [PubMed] [Google Scholar]
- Acute flaccid paralysis (AFP) surveillance, Global polio Eradication Initiative [database on the Internet]. World Health Organization. Available at: http://www.polioeradication.org/Dataandmonitoring/Surveillance.aspx.accessed December18, 2011.
- Barzegar M, Dastgiri S, Karegarmaher MH, et al. Epidemiology of childhood Guillan-Barre syndrome in the north west of Iran. BMC Neurol 2007;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoula S, Giannopoulos S, Sarmas I, et al. Guillain-Barre syndrome in northwest Greece. Acta Neurol Scand 2007;115(3):167–73. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Laboratory Accreditation Is Revised. Polio Lab Network, Quarterly Update. Vaccines and other Biologicals. 2000; V1: 1–4 Available at: http://apps.who.int/immunization_monitoring/61.pdf.accessed July16, 2012. [Google Scholar]
- World Health Organization. Dept. of Immunization Vaccines and Biologicals. Polio laboratory manual.4th ed. Geneva: World Health Organization; 2004. [Google Scholar]


