Abstract
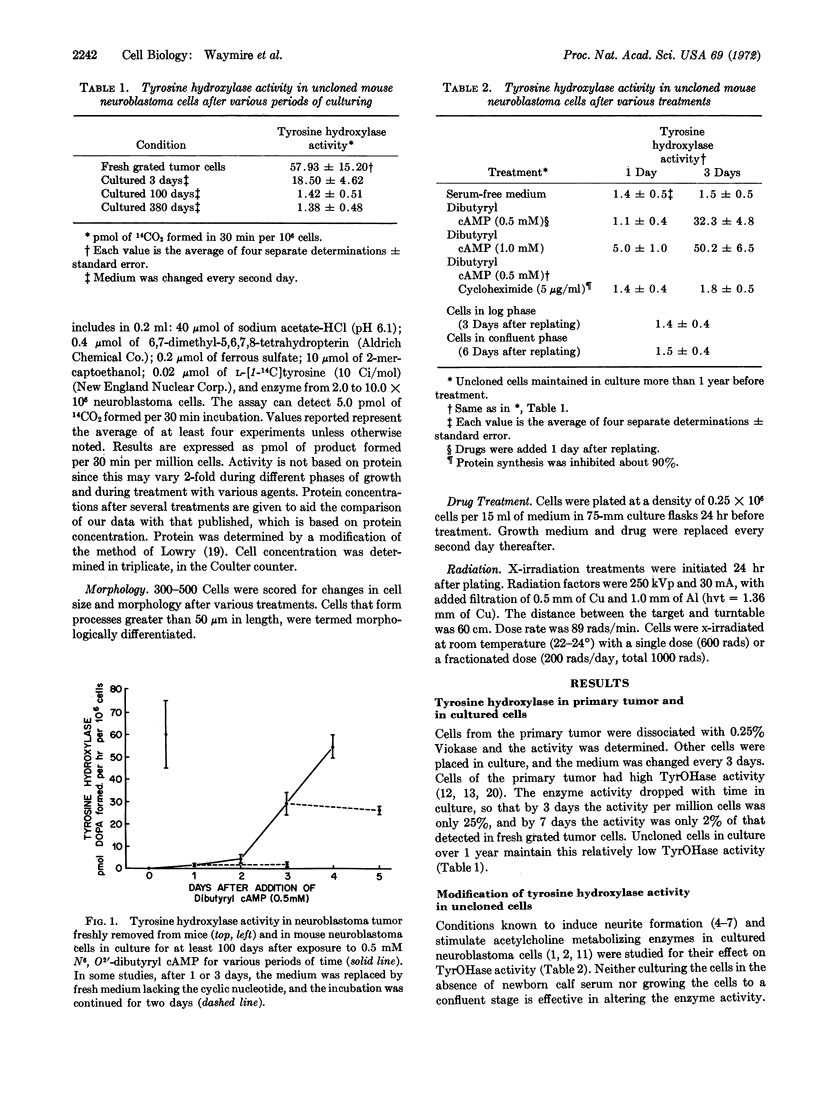
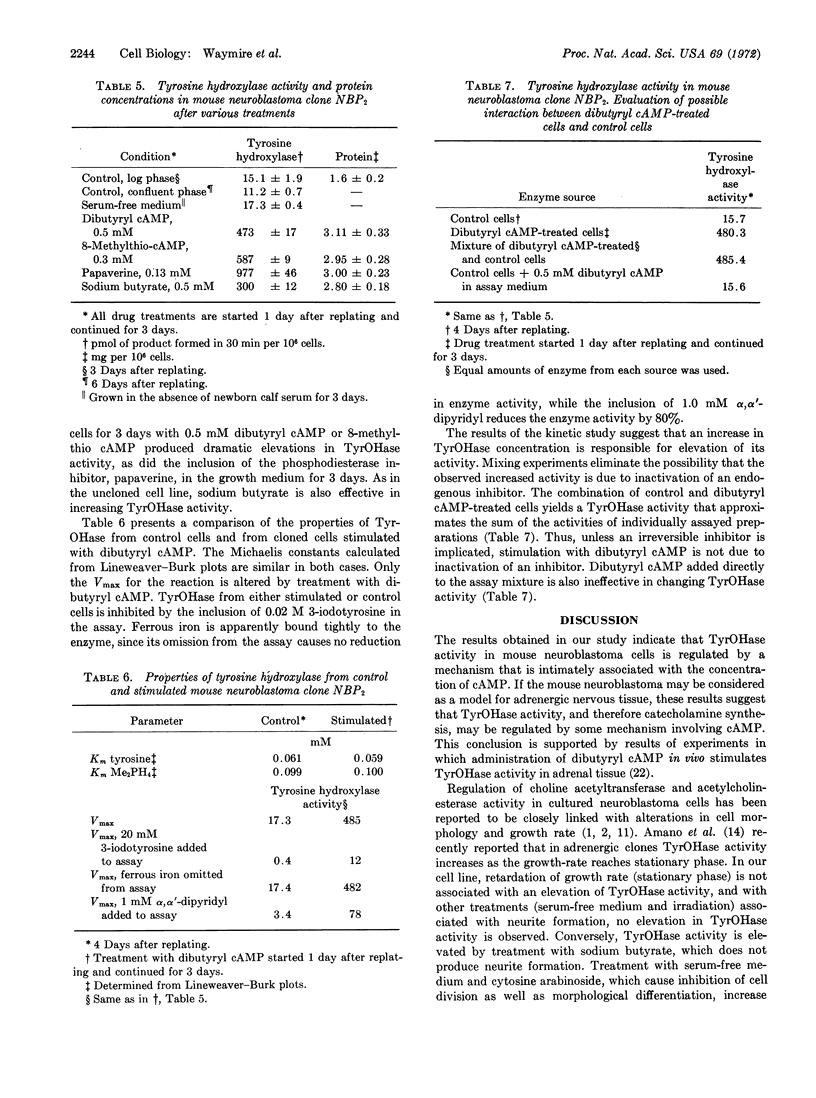
Mouse neuroblastoma cells in culture have been used as a model for the study of the mechanism by which activities of tyrosine hydroxylase (EC 1.14.3.a) are regulated in sympathetic tissue. The activity of tyrosine hydroxylase in cultured cells drops to barely detectable activities after 1 week and remains low for months in culture in the uncloned cell line of neuroblastoma. Activity in an adrenergic clone isolated from the uncloned line has about 20% of the activity of the fresh grated tumor cell. N6, O2′-dibutyryl adenosine 3′:5′-cyclic monophosphate causes a concentration and time-dependent increase in enzyme activity in both the cloned and uncloned cell lines. Enzyme activity is elevated by other stable analogs of adenosine 3′:5′-cyclic monophosphate, notably the N6-monobutyryl, 8-aminomethyl, and 8-methylthio derivatives of the cyclic nucleotide; by the inhibitor of cyclic nucleotide phosphodiesterase, papaverine; and by sodium butyrate. Changes in cell morphology and tyrosine hydroxylase activity are shown not to be necessarily related.
Keywords: adrenergic clones, cell morphology, x-irradiation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Anagoste B., Goldstein M. N. Tyramine-H3: deaminated metabolites in neuroblastoma tumors and in continuous cell line of a neuroblastoma. Science. 1968 May 17;160(3829):767–768. doi: 10.1126/science.160.3829.767. [DOI] [PubMed] [Google Scholar]
- Harris A. J., Dennis M. J. Acetylcholine sensitivity and distribution on mouse neuroblastoma cells. Science. 1970 Feb 27;167(3922):1253–1255. doi: 10.1126/science.167.3922.1253. [DOI] [PubMed] [Google Scholar]
- Kates J. R., Winterton R., Schlessinger K. Induction of acetylcholinesterase activity in mouse neuroblastoma tissue culture cells. Nature. 1971 Jan 29;229(5283):345–347. doi: 10.1038/229345a0. [DOI] [PubMed] [Google Scholar]
- Kvetñanský R., Gewirtz G. P., Weise V. K., Kopin I. J. Effect of dibutyryl cyclic-AMP on adrenal catecholamine-synthesizing enzymes in repeatedly immobilized hypophysectomized rats. Endocrinology. 1971 Jul;89(1):50–55. doi: 10.1210/endo-89-1-50. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson P., Ruffner W., Nirenberg M. Neuronal tumor cells with excitable membranes grown in vitro. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1004–1010. doi: 10.1073/pnas.64.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Carlson K., Klebe R., Ruddle F., Rosenbaum J. Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Jan;65(1):129–136. doi: 10.1073/pnas.65.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N. Effect of dopamine and 6-hydroxydopamine on mouse neuroblastoma cells in vitro. Cancer Res. 1971 Oct;31(10):1457–1460. [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Vernadakis A. Morphological and biochemical study in x-ray- and dibutyryl cyclic AMP-induced differentiated neuroblastoma cells. Exp Cell Res. 1972 Jan;70(1):27–32. doi: 10.1016/0014-4827(72)90177-2. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. X-ray-induced morphological differentiation of mouse neuroblastoma cells in vitro. Nature. 1971 Dec 24;234(5330):471–473. doi: 10.1038/234471a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Zambernard J., Lasher R., VanWoert M. H. Transmission of mouse neuroblastoma by a cell-free extract. Nature. 1970 Dec 5;228(5275):997–999. doi: 10.1038/228997a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N., Vandeventer L., De Francesco L., Friedkin M. E. Regulation of the synthesis of choline-O-acetyltransferase and thymidylate synthetase in mouse neuroblastoma in cell culture. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1436–1440. doi: 10.1073/pnas.68.7.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Triner L., Vulliemoz Y., Schwartz I., Nahas G. G. Cyclic phosphodiesterase activity and the action of papaverine. Biochem Biophys Res Commun. 1970 Jul 13;40(1):64–69. doi: 10.1016/0006-291x(70)91046-6. [DOI] [PubMed] [Google Scholar]
- Waymire J. C., Bjur R., Weiner N. Assay of tyrosine hydroxylase by coupled decarboxylation of DOPA formed from 1- 14 C-L-tyrosine. Anal Biochem. 1971 Oct;43(2):588–600. doi: 10.1016/0003-2697(71)90291-0. [DOI] [PubMed] [Google Scholar]
- Weiner N. Regulation of norepinephrine biosynthesis. Annu Rev Pharmacol. 1970;10:273–290. doi: 10.1146/annurev.pa.10.040170.001421. [DOI] [PubMed] [Google Scholar]


