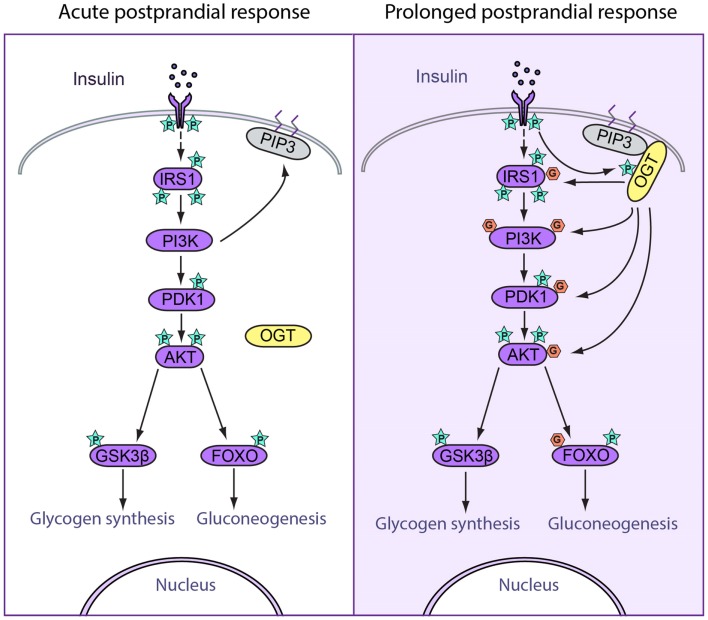
Figure 1.
Spatiotemporal regulation of feeding response by O-GlcNAcylation. (Left) Acute postprandial response. During early insulin signaling, OGT remains in the cytosol. Insulin binds to insulin receptor (IR) and triggers its autophosphorylation. Phosphorylation of IR recruits IRS1 to be phosphorylated, after which IRS1 binds to PI3K. PI3K catalyzes the production of PIP3, which recruits PDK1 to be phosphorylated and activated. Activated PDK1 phosphorylates and activates AKT, which further phosphorylates and activated downstream targets, including GSK3β and FOXO, which enhances glycogen synthesis and suppresses gluconeogenesis. (Right) Prolonged postprandial response. The insulin signaling pathway needs to be attenuated after a period of stimulation in order to maintain homeostasis. O-GlcNAcylation of insulin signal proteins contributes to the attenuation of the pathway. During prolonged insulin signaling, OGT translocates to the plasma membrane and binds with PIP3 through the PIP3-binding domain. OGT is then phosphorylated and activated by IR. Activated OGT O-GlcNAcylates key insulin signaling proteins including IRS1, PI3K, PDK1, and AKT, antagonizing the activation by phosphorylation on these proteins. These events lead to decreased glycogen synthesis and increased gluconeogenesis.