Abstract
Objective
Revision total hip arthroplasty (THA) is associated with increased cost, morbidity, and technical challenge compared to primary THA. A better understanding of the risk factors for early revision is needed to inform strategies to optimize patient outcomes.
Methods
207,256 patients who underwent primary THA between 1997–2005 in California and New York were identified from statewide databases. Unique patient identifiers were used to identify early revision THA (<10 years from index procedure). Patient characteristics (demographics, comorbidities, insurance type, preoperative diagnosis), community characteristics (education level, poverty, population density), and hospital characteristics (annual THA volume, bed size, teaching status) were evaluated using multivariable regression to determine risk factors for early revision.
Results
The probabilities of undergoing early aseptic revision and early septic revision were 4% and less than 1% at 5 years, respectively. Women were 29% less likely than men to undergo early septic revision (p<0.001). Patients with Medicaid and Medicare were 91% and 24%, respectively, more likely to undergo early septic revision than privately-insured patients (p=0.01; p<0.001). Hospitals performing <200 THA annually had a 34% increased risk of early aseptic revision compared to hospitals performing >400 THA annually (p<0.001).
Conclusion
A number of identifiable factors, including younger age, Medicaid, and low hospital volume increase the risk of undergoing early revision THA. Patient-level characteristics distinctly affect the risk of revision within 10 years, particularly if due to infection. Our findings reinforce the need for continued investigation of the predictors of early failure following THA.
The utilization of total hip arthroplasty (THA) is growing rapidly, with a projected demand of 572,000 cases per year by 2030 (1). While the relative proportion of revisions is expected to remain at a similar level in the foreseeable future, the total number of revision THA performed is projected to double by 2026 (1) with associated charges estimated to exceed 4 billion USD by 2015(2). Late revision is a foreseeable reality for many patients given the finite longevity of implants, rising use of THA in younger patients, and the increasing cumulative demand being placed on implants (3, 4). However, revisions that occur earlier than expected may indicate poor quality of care and represent an area for improvement. Strategies are needed to decrease the frequency of early revision, particularly as health care resources become increasingly constrained and efforts to improve the quality and efficiency of care are emphasized (5).
The substantial THA revision burden [revisions were 17.5% of all THA cases from 1990 to 2002] (6) indicates that opportunities for improvement exist. While aseptic loosening, instability, and infection are the leading causes for revision THA (7–9), increasing attention is being directed to the influence of patient-related characteristics on outcomes following THA. Allen et al demonstrated that patient socioeconomic characteristics were more predictive of postoperative thigh pain, dissatisfaction, and Harris hip scores than implant characteristics (10). Although an earlier systematic review revealed a lack of consistent evidence (11), recent rigorously conducted investigations demonstrated a notable influence of patient characteristics on prognosis following primary THA (12, 13). In addition to patient characteristics, a number of hospital and community-related variables may contribute to the frequency of revision THA. A detailed understanding of these structural and “process factors” (14) and community variables may provide additional insight into the circumstances surrounding revision THA and represent opportunities for systems-based improvements in the delivery of care.
In the current investigation, we used administrative databases from two states (California and New York) to evaluate the influence of patient, community, and hospital-related characteristics on the risk of early revision THA. We hypothesized that early revision THA is more common in younger patients, in patients from communities with low socioeconomic status, and in patients who undergo primary THA at low volume hospitals.
METHODS
Study Population and Data Sources
The New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS) collects information on all discharges from non-federal acute care hospitals in New York State. We used SPARCS data from 1997 to 2005 because recording of unique patient identifiers (UPID) for patients began in 1997. The California Office of Statewide Health Planning and Development (OSHPD) maintains a similar database from which we used data from 1997 to 2005. Data from index procedures in 2005 (with follow-up until December 31, 2006) were the most recently available records when we conducted this investigation.
The American Hospital Association (AHA) Annual Survey provides information on hospital characteristics. These data were linked to the NY and CA discharge data using AHA hospital identifiers and Healthcare Utilization Project (HCUP) linkage files, enabling us to identify teaching status, bed size, and rurality. US Census data were used to estimate community poverty and educational levels based on the patient’s residential zip code.
Definition of Total Hip Arthroplasty Cohort
The index cohort was defined as NY and CA residents undergoing a primary total hip arthroplasty (THA) (ICD-9-CM procedure code 81.51) from 1997 to 2005 with no diagnosis code indicating a prior hip replacement (ICD-9-CM V43.64). A total of 207,256 primary THA were eligible for this project after applying these criteria (123,600 [59.6%] in CA; 83,656 [40.4%] in NY).
Endpoint of Analysis
Revision THA was defined among patients identified in the index cohort as having one of the revision THA procedure codes (ICD-9-CM codes 00.70-00.73, 81.53) either on a subsequent day during the same admission (in-hospital revision prior to discharge) or in a subsequent admission within the study period. Given that 10-year implant survivorship for primary THA has exceeded 95% in long-term clinical series (15–18) and registry studies (19, 20), revision within 10 years was considered early.
Patients not undergoing revision THA were censored at the time of in-hospital death (in the index or a subsequent admission), or at the end of the study period (December 31, 2006), whichever came first. Due to concerns for out-of-hospital mortality, which is not captured in these data, we used Centers for Disease Control (CDC) life tables to estimate censoring date for patients not expected to live to the end of the follow-up period. Patients were also censored if they underwent a subsequent primary THA prior to their revision THA to minimize potential misclassification of revision laterality (21). This censoring only occurred at the time of any revision surgery. The median follow-up time for the study cohort was 3.8 years (interquartile range: 1.7 to 6.3 years).
Definitions of Predictors
Patient characteristics
Age, sex, race, primary surgical diagnosis, comorbidities, and insurance status were considered potential patient-level predictors of early revision THA. Race is a mandatory data field in California, but is a voluntarily reported field in New York. Race was defined as white, black, Hispanic, Asian/Pacific Islander, Native American, or other.
Surgical diagnosis was defined as osteoarthritis (OA), inflammatory arthritis (e.g., rheumatoid arthritis), trauma, avascular necrosis, or other based on the ICD-9-CM diagnosis fields. The “other” category was only used in cases where none of the aforementioned diagnoses were coded. In cases where a second diagnosis was coded in addition to OA, the non-OA diagnosis was given primacy. This was done to minimize the over-reporting of OA as the primary reason for THA.
Comorbidity scores were calculated using the Elixhauser comorbidity index (22). Payer (insurance) status was defined as private, Medicare, Medicaid, self-pay, or other.
Community and Institutional Characteristics
Community education level, household income, percentage below poverty level, and population density were estimated based on patient residential zip code using US Census Bureau data from the 2000 Census. Hospital THA volume was calculated for the four quarters prior to the quarter of the index surgery for each patient. Number of hospital beds and teaching status were identified using the AHA Annual Survey for each institution. The designation of the hospital as urban or rural was based on the Rural-Urban Commuting Area (RUCA) Codes (23).
Reason for Revision
The primary reason for revision was determined from review of the principal/admitting diagnosis coding at time of revision. ICD-9-CM codes were used to categorize reasons for revision as acute fracture, septic failure, aseptic failure, dislocation, and other (Supplementary Material).
Statistical Analysis
The effects of patient, community, and hospital characteristics on the likelihood of early revision THA were estimated using a multivariable generalized estimating equation (GEE). Separate models were constructed for aseptic and septic reasons for early revision THA. The GEE was used to account for the potential of clustering of patient characteristics within surgeons and hospitals. The probability of undergoing revision THA was calculated using Kaplan-Meier methods for the entire cohort, stratified on separate curves by sex (Figure 1) and reason for revision (aseptic or septic) (Figure 2), and adjusted for state due to differences in data collection methods. A competing risks analysis was used, with one type of revision event (septic or aseptic) precluding the observation of the other type of revision event.
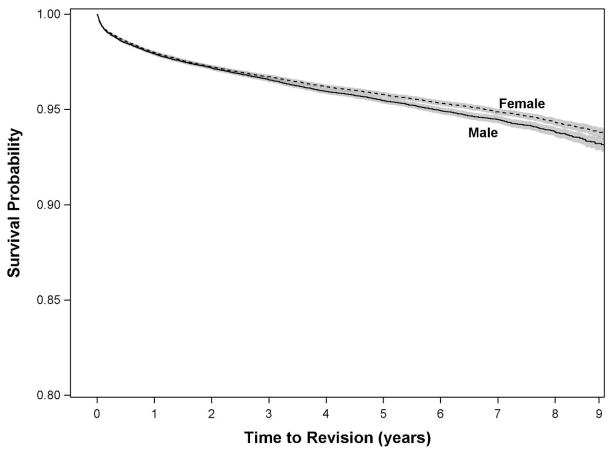
Figure 1.
Kaplan-Meier survivorship curve for undergoing revision total hip arthroplasty (all-causes) within 9 years of index surgery, stratified by sex.
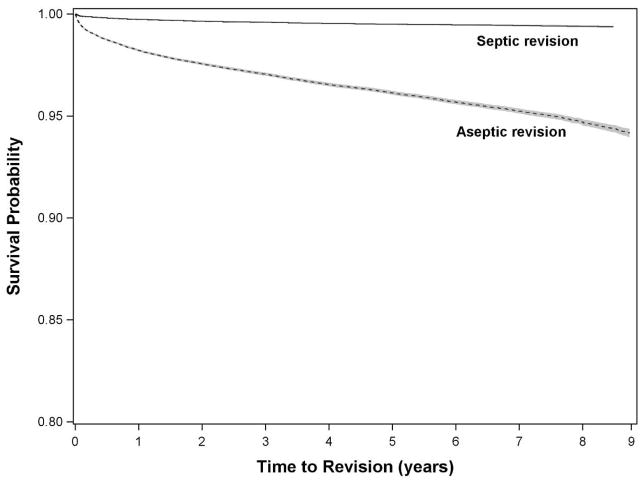
Figure 2.
Kaplan-Meier survivorship curve for undergoing revision total hip arthroplasty within 9 years of index surgery, stratified by reason for revision (aseptic or septic).
RESULTS
Patient demographics, community factors, and institution factors for all patients
The median age for patients undergoing primary THA was 68 years (interquartile range: 58 to 76 years). The majority (59.1%) of patients were between 50 and 75 years, male (58.3%), and white (85.1%) (Table 1). Medicare was the most common insurance type (56.8%), followed by private insurance (36.3%). Osteoarthritis was the most common primary diagnosis (77.0%), followed by avascular necrosis (8.4%) and fracture (7.0%).
Table 1.
Patient Demographics at the Time of Index THA (n=207,256)
| Patient Factors | Categories | # (%) |
|---|---|---|
| Age | <50 | 27,121(13.1) |
| 50–75 | 122,551(59.1) | |
| >75 | 57,584(27.8) | |
| Sex | Female | 86,431(41.7) |
| Male | 120,825(58.3) | |
| Race | White | 176,311(85.1) |
| Black | 10,792(5.2) | |
| Other | 10,660(5.1) | |
| Unknown | 9,493(4.6) | |
| Insurance Type | Medicare | 117,663(56.8) |
| Medicaid | 7,528(3.6) | |
| Private | 75,315(36.3) | |
| Self-Pay | 1,086(0.5) | |
| Other | 5,664(2.7) | |
| Indication for TJA | OA | 159,580(77.0) |
| IA | 8,652(4.2) | |
| AVN | 17,389(8.4) | |
| Congenital | 4,451(2.1) | |
| Fracture | 14,559(7.0) | |
| Neoplasm | 1,616(0.8) | |
| Other | 1,009(0.5) | |
| Comorbidities* | Congestive heart failure | 5,630(2.7) |
| Valvular disease | 8,525(4.1) | |
| Peripheral vascular disease | 3,302(1.6) | |
| Other neurological disorders | 4,579(2.2) | |
| COPD | 22,936(11.1) | |
| Diabetes | 18,319(8.8) | |
| Hypothyroidism | 19,362(9.3) | |
| Obesity | 13,473(6.5) | |
| Coagulopathy | 2,171 (1.0) | |
| Fluid & electrolyte disorders | 11,472(5.5) | |
| Depression | 9,014(4.3) | |
| Hypertension | 94,420(45.6) |
Percentages for comorbidities do not sum to 100 due to patients having multiple comorbidities.
Primary THA was performed most commonly (55.0%) in low volume centers (≤200/year), with 15.0% performed in high-volume centers (>400/year) (Table 2). THA was more commonly performed in non-teaching hospitals (74.3%) and urban hospitals (93.9%) (Table 2). The median percentage of people with a college degree in the patients’ communities was 26.0%, the median household income was $39,750, and the median percentage of people below the poverty level in the patients’ communities was 7.0% (Table 2).
Table 2.
Hospital & Community Factors from the Index THA (n=207,256)
| Categories | # (%) | |
|---|---|---|
| Hospital Factors | ||
| Annual THA Volume | ≤200 | 113,904(55.0) |
| 201–400 | 62,198(30.0) | |
| 400+ | 31,154(15.0) | |
| Bed Size | <50 | 3,743(1.8) |
| 50–200 | 60,881(29.4) | |
| 200–400 | 90,460(43.7) | |
| 400+ | 52,172(25.2) | |
| Teaching Hospital | Teaching | 53,236(25.7) |
| Non-teaching | 154,020(74.3) | |
| Urban/Rural Hospital | Urban | 194,612(93.9) |
| Rural | 12,644(6.1) | |
| Community Factors | ||
| Education (% college graduate) | Q1 | 17.0% |
| Median | 26.0% | |
| Q3 | 40.0% | |
| Median Household Income | Q1 | $28,019 |
| Median | $39,750 | |
| Q3 | $57,333 | |
| Poverty Level (%) | Q1 | 5.0% |
| Median | 7.0% | |
| Q3 | 12.0% | |
| Population Density (per sq mile) | Q1 | 540.86 |
| Median | 2,882.87 | |
| Q3 | 7,374.02 | |
Probability of Undergoing Early Revision (Aseptic and Septic)
Based on Kaplan-Meier survivorship analysis, the probability of undergoing early aseptic revision was 4% (survival rate 0.961 [0.960,0.962]) and 6% (survival rate 0.942 [0.939,0.944]) at 5 and 9 years, respectively (Figure 2). The probability of undergoing early revision due to infection was less than 1% at 5 (survival rate 0.9949 [0.9946, 0.9953]) and 9 years (survival rate 0.9936 [0.9930, 0.9942]), respectively (Figure 2).
Patient Demographics and Early Revision (Aseptic and Septic)
After adjusting for all other patient, community, and hospital characteristics, patients 50 to 75 years of age and >75 years had hazard ratios (HR) of 0.84 (95% CI: 0.78, 0.90) and 0.63 (95% CI: 0.57, 0.69), respectively, for aseptic revision compared to patients <50 years. The protective effect of increasing age was more pronounced for the risk of septic revision (Table 4). Women were less likely than men to undergo early septic revision (HR 0.71; 95% CI: 0.62, 0.82), but sex did not affect the risk of early aseptic revision. Compared to white patients, black patients were not at increased risk for early septic or aseptic revision.
Table 4.
Community & Hospital Characteristics Associated with Early Revision THA (7,945 revisions among 207,256 patients).
| Septic Revision | Aseptic Revision | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | |
| Hospital Factors | ||||||
| THA volume | ||||||
| ≤200 v. >400 | 1.22 | (0..81,1.84) | 0.342 | 1.33 | (1.16,1.52) | <0.001 |
| 201–400 v. >400 | 1.14 | (0.75,1.74) | 0.488 | 1.42 | (1.24,1.64) | <0.001 |
| Bed size | ||||||
| 51–200 v. <50 | 1.93 | (0.95,3.91) | 0.070 | 0.99 | (0.82,1.18) | 0.869 |
| 200–400 v. <50 | 1.96 | (0.96,4.00) | 0.064 | 1.06 | (0.88,1.27) | 0.570 |
| >400 v. <50 | 2.13 | (1.03,4.40) | 0.042 | 1.19 | (0.98,1.44) | 0.083 |
| Teaching hospital | 1.06 | (0.81,1.40) | 0.668 | 1.00 | (0.90,1.11) | 0.927 |
| Interaction Variables | ||||||
| THA Volume 201–400 * Teaching Hospital | 0.99 | (0.73,1.34) | 0.747 | 0.97 | (0.86,1.09) | 0.095 |
| THA Volume >400 * Teaching Hospital | 0.80 | (0.58,1.13) | 0.946 | 0.79 | (0.70,0.90) | 0.517 |
| Urban v. Rural | 0.80 | (0.62,1.05) | 0.113 | 1.06 | (0.95,1.19) | 0.302 |
| Community Factors | ||||||
| Education | ||||||
| 50th v. 25th | 0.83 | (0.69,1.00) | 0.053 | 0.97 | (0.91,1.05) | 0.480 |
| 75th v. 25th | 0.85 | (0.70,1.04) | 0.116 | 1.07 | (0.99,1.15) | 0.075 |
| 100th v. 25th | 0.83 | (0.67,1.03) | 0.088 | 1.06 | (0.98,1.14) | 0.173 |
| Poverty | ||||||
| 50th v. 25th | 1.07 | (0.88,1.31) | 0.491 | 1.02 | (0.95,1.09) | 0.596 |
| 75th v. 25th | 1.02 | (0.82,1.26) | 0.878 | 1.06 | (0.99,1.14) | 0.108 |
| 100th v. 25th | 1.21 | (0.97,1.51) | 0.088 | 1.02 | (0.95,1.11) | 0.560 |
Medicare patients were more likely to undergo aseptic (HR 1.07; 95% CI: 1.01, 1.13) and septic (HR 1.24; 95% CI: 1.05, 1.47) early revision THA, respectively, than patients with private insurance after adjustment for all other characteristics, including age. Medicaid patients were 91% more likely (HR 1.91; 95% CI: 1.45, 2.51) to undergo early septic revision compared to privately insured patients, but were not at increased risk for early aseptic revision.
Patients with primary diagnoses other than osteoarthritis were generally at increased risk for both aseptic and septic early revision THA (Table 3). These risks reached the greatest magnitude for early septic revision. Patients with hypothyroidism and those with depression were at increased risk for both aseptic and septic early revision. Comorbid diagnoses of diabetes (HR 1.44; 95% CI: 1.18, 1.76), chronic pulmonary disease (HR 1.30; 95% CI: 1.07, 1.56), obesity (HR 1.68; 95% CI: 1.34, 2.09), and fluid/electrolyte disorders (HR 1.34; 95% CI: 1.04, 1.72) significantly increased the risk for early septic (but not aseptic) revision (Table 3).
Table 3.
Patient Characteristics Associated with Early Revision THA (7,945 revisions among 207,256 patients).
| Septic Revision (n=889) | Aseptic Revision (n=7,056) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | |
| Age | ||||||
| 50–75 v. <50 | 0.78 | (0.64,0.95) | 0.015 | 0.84 | (0.78,0.90) | <0.001 |
| >75 v. <50 | 0.51 | (0.39,0.66) | <0.001 | 0.63 | (0.58,0.69) | <0.001 |
| Female | 0.71 | (0.62,0.82) | <0.001 | 0.98 | (0.93,1.03) | 0.414 |
| Race | ||||||
| Black v. White | 1.08 | (0.83,1.40) | 0.570 | 1.08 | (0.98,1.20) | 0.140 |
| Other v. White | 0.85 | (0.63,1.15) | 0.300 | 0.79 | (0.70,0.89) | <0.001 |
| Unknown v. White | 0.59 | (0.39,0.89) | 0.012 | 0.82 | (0.71,0.93) | 0.003 |
| Insurance | ||||||
| Medicare v. Private | 1.24 | (1.05,1.47) | 0.011 | 1.07 | (1.01,1.13) | 0.022 |
| Medicaid v. Private | 1.91 | (1.45,2.51) | <0.001 | 1.12 | (0.10,1.27) | 0.068 |
| Self-Pay v. Private | 2.19 | (1.15,4.12) | 0.015 | 0.88 | (0.62,1.25) | 0.494 |
| Other v. Private | 1.54 | (1.10,2.16) | 0.012 | 1.14 | (1.00,1.31) | 0.049 |
| Surgical indication | ||||||
| IA v. OA | 1.59 | (1.20,2.08) | 0.001 | 1.23 | (1.11,1.37) | 0.001 |
| AVN v. OA | 1.26 | (1.01,1.57) | 0.045 | 1.21 | (1.11,1.31) | <0.001 |
| Congenital v. OA | 0.64 | (0.35,1.17) | 0.150 | 1.17 | (1.01,1.36) | 0.041 |
| Acute Fracture v. OA | 1.85 | (1.48,2.31) | <0.001 | 1.15 | (1.05,1.27) | 0.003 |
| Neoplasm v. OA | 1.79 | (1.01,3.17) | 0.046 | 1.13 | (0.88,1.45) | 0.359 |
| Other v. OA | 2.93 | (1.72,5.00) | <0.001 | 1.88 | (1.49,2.38) | <0.001 |
| Elixhauser Comorbidities | ||||||
| Congestive heart failure | 0.98 | (0.67,1.44) | 0.919 | 0.89 | (0.76,1.04) | 0.140 |
| Valvular disease | 0.84 | (0.57,1.22) | 0.355 | 0.93 | (0.81,1.06) | 0.270 |
| Peripheral vascular disease | 0.97 | (0.58,1.62) | 0.893 | 0.84 | (0.68,1.03) | 0.097 |
| Other neurological disorders | 0.96 | (0.63,1.46) | 0.836 | 1.01 | (0.86,1.19) | 0.870 |
| Chronic pulmonary disease | 1.30 | (1.07,1.56) | 0.007 | 1.08 | (1.00,1.16) | 0.052 |
| Diabetes | 1.44 | (1.18,1.76) | 0.001 | 0.92 | (0.84,1.01) | 0.066 |
| Hypothyroidism | 1.33 | (1.06,1.66) | 0.013 | 1.09 | (1.01,1.19) | 0.032 |
| Coagulopathy | 1.56 | (0.96,2.53) | 0.072 | 0.94 | (0.74,1.19) | 0.606 |
| Obesity | 1.68 | (1.34,2.10) | <0.001 | 0.91 | (0.82,1.01) | 0.073 |
| Fluid and electrolyte disorders | 1.34 | (1.04,1.72) | 0.025 | 0.97 | (0.87,1.08) | 0.536 |
| Depression | 1.50 | (1.14,1.98) | 0.004 | 1.41 | (1.27,1.57) | <0.001 |
| Hypertension | 1.09 | (0.94,1.25) | 0.255 | 1.01 | (0.96,1.06) | 0.786 |
Community Factors, Institutional Factors, and Early Revision (Aseptic and Septic)
The lowest THA volume hospitals (≤200 annual THA cases) exhibited an increased risk for early aseptic revision (HR 1.33; 95% CI: 1.16,1.52) compared to the highest THA volume hospitals (>400 annual THA cases). The risk of early aseptic revision was also increased (HR 1.42; 95% CI: 1.24,1.64) in the mid-volume (201–400 annual THA cases) compared to the highest THA volume hospitals. No association was found between hospital THA volume and the risk of early septic revision.
There was an increased risk for early septic revision in the hospitals with the largest bed capacity (>400 beds) (HR 2.13; 95% CI: 1.03, 4.40) compared to hospitals with the lowest bed capacity (<50 beds) (Table 4). No associations were found between education level or poverty level of the surrounding community and risk for early revision THA (Table 4).
DISCUSSION
In addition to being technically challenging (24–26), revision THA is associated with a greater risk of post-operative complications (27), subsequent re-revision (28), and results that are less predictable than primary THA (29, 30). While the majority of previous investigations on outcomes following THA have focused on the effects of surgical technique and implant design (10), detailed evaluation of patient, community, and hospital-related characteristics can be used to develop strategies to decrease the frequency of revision THA. We found that patient age, insurance type, preoperative diagnosis, and hospital volume were all significantly related to an increased risk for early revision THA. These effects were particularly pronounced in sub-group analysis of patients who underwent early revision of an infected implant. The increased risk of revision at the lowest volume hospitals is not surprising given previous demonstrations of volume-outcome benefit (31–33). When considering risk factors for early septic revision, the findings of increased risk in hospitals with >400 beds and no difference in risk at high volume hospitals indicate that specialty hospitals (with high volume and relatively low bed numbers) may be beneficial in protecting against infection. We found that patients under 50 years of age are at significantly higher risk for revision, which corroborates national registry findings from Norway (34) and Sweden (35) as well as a recent systematic review (11). Increased activity levels in younger patients may place greater mechanical demands on the implant, potentially predisposing the THA to early failure (3, 4). The influence of sex on outcomes after THA is still being debated. Our results indicate no difference in the risk for aseptic early revision and a significantly decreased risk in female patients for septic early revision. These findings are somewhat contradictory to a previous systematic review that showed no effect of sex on revision risk (11) and Inacio’s recent findings of an increased risk of all-cause and aseptic revision in women (13). The inconsistency in the literature is likely due to differences in definitions of THA failure, data sources, and statistical analysis methods, but also highlights the need for a national total joint arthroplasty registry that spans multiple payers and includes patient characteristic information beyond what is available in administrative datasets.
The influence of community factors on risk of revision after primary THA has not been well described (11, 36). Our results are consistent with the only other epidemiologic study that evaluated the influence of sociodemographic risk factors on risk of revision (37). Agabiti et al’s review of Italian registry data showed no effect of income level on risk of revision. However, Allen’s single center study demonstrated a negative influence of ethnicity, education level, poverty level, and income on clinical outcomes after THA (10). The discrepancy between our findings and Allen’s may be attributable to differences in data collection and chosen outcomes.
Unlike clinical outcome measures, our primary outcome (revision) is contingent on patients having access to a surgeon that is able to recognize the need for further surgery and willing to perform the revision. The relationship between sociodemographic risk factors and clinical outcomes (such as those used by Allen) may be different than the relationship of the same risk factors with risk of revision, mainly because of the complexity of proceeding from a painful postoperative THA to actually having a revision. Additionally, we cannot evaluate individual income and education with administrative data. This leaves us only with an indirect assessment using poverty and education levels of the surrounding community. Lastly, Medicaid eligibility is based on income level, therefore lessening any potential association of poverty with early revision after controlling for insurance type in our multivariable model. Insurance status was not included in Allen’s regression model (10).
Previous single-center series have demonstrated that Medicaid patients are at increased risk for inferior functional outcomes compared to those with Medicare or private insurance (38–40). Our population-based results complement the work of prior authors by demonstrating a 91% increased risk of early septic revision in Medicaid patients compared to those with private insurance, supporting the notion that this patient group is particularly vulnerable to poor outcomes after THA. The potential relative risk of Medicare insurance is less well understood. Medicare patients in our study had a 24% increase in risk for early septic revision compared to privately insured patients, even after adjustment for age and other characteristics. To date, no other studies have demonstrated an increased risk of complications in the Medicare population compared to privately insured patients. The novelty of these insurance-based findings indicates that further investigation is needed to elucidate the influence of different insurance types on complications after THA. This is particularly salient as Medicare enrollment grows and as implementation of the Affordable Care Act expands Medicaid eligibility.
Our study provides supportive data for preoperative risk counseling prior to THA, particularly related to the primary surgical diagnosis and medical comorbidities. As in prior reports (41, 42), patients with primary diagnoses of inflammatory arthritis, avascular necrosis (AVN), and fracture had significantly higher rates of early revision compared to patients with osteoarthritis. Our analysis demonstrates that a comorbid diagnosis of hypothyroidism increases the risk for both aseptic and septic early revision. This may be related to metabolic bone disease associated with the disease (43), especially in light of findings that poor bone quality is related to complications after THA (44). Our findings indicate that a diagnosis of depression also increases the risk for both aseptic and septic early revision, complementing data from the Swedish national registry that patients with depression have less satisfaction and less pain relief following THA (45). Focused analysis of the patients who underwent early revision due to infection indicates that comorbidities play a larger role in determining the risk of early septic failure after THA. Diabetes, obesity, and chronic pulmonary disease increased the risk for early revision due to infection by 43%, 67%, and 29%, respectively, which support the findings of previous population-based studies with shorter timeframes (12, 46) and from single payers (28). This information may be particularly helpful to clinicians in counseling patients with these comorbidities about their increased risk for infection after THA.
Our study has additional limitations, specifically those inherent to the use of administrative databases in health services research. We were unable to capture complications that may have occurred outside of the state where the index THA was performed. We attempted to minimize the effect of this limitation by including only residents from CA or NY in our cohort, as out of state residents may be more likely to seek follow-up care outside of CA or NY. Additionally, our administrative data rely on consistent and accurate recording of complication codes across varying practice settings. Our results must be interpreted with caution due to limitations in our data on reason for revision. Because the detailed ICD-9-CM coding of reason for revision was not implemented until the last 3 months of our timeframe (introduced October 1, 2005; study period from 1997 to 2005), we instead categorized the admitting diagnoses as fracture, septic failure, aseptic failure, dislocation, and other. While this method is not as precise as using the updated codes, the rate of infection as the reason for revision in our cohort (11.2%) is similar to that reported in a study using the updated coding (14.8%) (8). The uncertainties of this aspect of our data make it difficult to draw conclusions about reasons for early revision, but the associations we report can provide insight to shape future prospective research. Another limitation of our data is the inability to include surgeon characteristics in our analysis because these data are unavailable in California. While our finding that hospital volume is related to early revision is consistent with the literature (33), surgeon characteristics deserve additional attention because their influence on outcomes after THA may be different than that of hospital characteristics. Additionally, as mentioned earlier, our reliance on administrative data does not allow us to evaluate the association between individual income and education levels on risk for early revision. We are only able to evaluate the association of the surrounding community’s poverty and education levels with the risk of early revision after THA. Furthermore, our use of administrative data limits us to studying only those patients who are utilizing health services and inherently excludes patients who cannot access the same services. The latter group deserves further study, as Lavernia et al reported that less than 15% of patients with Medicaid coverage were able to obtain outpatient consultation for end-stage hip or knee arthritis in a densely populated urban area (47). Lastly, we are unable to measure severity of disease, both for the primary arthritic process (such as the extent of joint destruction or malalignment) and for comorbidities (such as extent of obesity or control of diabetes). Despite these drawbacks, our analysis of administrative data from CA and NY expands upon the current knowledge of complications after THA. We have built upon a previous investigation of short-term complications following THA that used the same database in CA (46) by following patients in two states (CA and NY) for up to 9 years to monitor for the occurrence of revision. Our use of statewide data from two diverse states allows us to include all age groups and payer types, which is a limitation of prior studies from the United States that included only patients with Medicare (14, 48) or a large single payer (49). The increased diversity of age and payer mix provides new information about the circumstances surrounding early revision THA that has not been previously reported.
In our population-based investigation, we have demonstrated the influence of patient- and hospital-related characteristics on the risk for early revision THA. Our findings reinforce the need for continued investigation of the variables related to the delivery of health care. It will be increasingly important to identify new strategies to optimize outcomes after THA in view of growing utilization and greater emphasis on quality.
SIGNIFICANCE AND INNOVATIONS.
Among 207,256 patients who underwent primary THA in California and New York over an 8-year period, the 5-year probability of undergoing revision THA is less than 1% if due to infection and 4% if due to non-infection causes.
Medicaid patients are at a 91% increased risk for early septic failure after THA compared to privately-insured patients.
Women have a 29% decreased risk for early septic failure after THA compared to men.
We provide population-based, multi-payer data from the United States that corroborate prior single center series and registry studies from other countries. Continued investigation of the variables related to the delivery of arthroplasty is needed to identify new strategies to optimize outcomes after THA.
Acknowledgments
Funding: This research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grants RC1 AR058280-01 (SL) and T32-AR07281 (CJD), as well as the Agency for Healthcare Research and Quality grant U18-HS16075 (SL).
Footnotes
Conflict of Interest: There was no other financial support or other benefits from commercial sources for this work. The authors do not have any other financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
REFERNCES
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007 Apr;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007 Oct;89(Suppl 3):144–51. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 3.Dorr LD, Takei GK, Conaty JP. Total hip arthroplasties in patients less than forty-five years old. J Bone Joint Surg Am. 1983 Apr;65(4):474–9. [PubMed] [Google Scholar]
- 4.Broos P, Fourneau I. Host factors that affect outcome of total hip arthroplasty. Lancet. 2000 Apr 29;355(9214):1479–80. doi: 10.1016/S0140-6736(00)02159-0. [DOI] [PubMed] [Google Scholar]
- 5.Lansky D, Nwachukwu BU, Bozic KJ. Using financial incentives to improve value in orthopaedics. Clin Orthop Relat Res. 2012 Apr;470(4):1027–37. doi: 10.1007/s11999-011-2127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005 Jul;87(7):1487–97. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004 Dec;429(429):188–92. doi: 10.1097/01.blo.0000150126.73024.42. [DOI] [PubMed] [Google Scholar]
- 8.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009 Jan;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 9.Dobzyniak M, Fehring TK, Odum S. Early failure in total hip arthroplasty. Clin Orthop Relat Res. 2006 Jun;447:76–8. doi: 10.1097/01.blo.0000203484.90711.52. [DOI] [PubMed] [Google Scholar]
- 10.Allen Butler R, Rosenzweig S, Myers L, Barrack RL. The Frank Stinchfield Award: the impact of socioeconomic factors on outcome after THA: a prospective, randomized study. Clin Orthop Relat Res. 2011 Feb;469(2):339–47. doi: 10.1007/s11999-010-1519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santaguida PL, Hawker GA, Hudak PL, Glazier R, Mahomed NN, Kreder HJ, et al. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg. 2008 Dec;51(6):428–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Bozic KJ, Lau E, Ong K, Chan V, Kurtz S, Vail TP, et al. Risk Factors for Early Revision After Primary Total Hip Arthroplasty in Medicare Patients. Clin Orthop Relat Res. 2013 May 29; doi: 10.1007/s11999-013-3081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inacio MC, Ake CF, Paxton EW, Khatod M, Wang C, Gross TP, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013 Mar 25;173(6):435–41. doi: 10.1001/jamainternmed.2013.3271. [DOI] [PubMed] [Google Scholar]
- 14.Heck DA, Melfi CA, Mamlin LA, Katz BP, Arthur DS, Dittus RS, et al. Revision rates after knee replacement in the United States. Med Care. 1998 May;36(5):661–9. doi: 10.1097/00005650-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Archibeck MJ, Berger RA, Jacobs JJ, Quigley LR, Gitelis S, Rosenberg AG, et al. Second-generation cementless total hip arthroplasty. Eight to eleven-year results. J Bone Joint Surg Am. 2001 Nov;83-A(11):1666–73. doi: 10.2106/00004623-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Engh CA, Jr, Culpepper WJ, 2nd, Engh CA. Long-term results of use of the anatomic medullary locking prosthesis in total hip arthroplasty. J Bone Joint Surg Am. 1997 Feb;79(2):177–84. [PubMed] [Google Scholar]
- 17.Clohisy JC, Harris WH. The Harris-Galante porous-coated acetabular component with screw fixation. An average ten-year follow-up study. J Bone Joint Surg Am. 1999 Jan;81(1):66–73. doi: 10.2106/00004623-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Milosev I, Kovac S, Trebse R, Levasic V, Pisot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012 Oct 3;94(19):1756–63. doi: 10.2106/JBJS.J.01858. [DOI] [PubMed] [Google Scholar]
- 19.Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000 Aug;71(4):337–53. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- 20.Hooper GJ, Rothwell AG, Stringer M, Frampton C. Revision following cemented and uncemented primary total hip replacement: a seven-year analysis from the New Zealand Joint Registry. J Bone Joint Surg Br. 2009 Apr;91(4):451–8. doi: 10.1302/0301-620X.91B4.21363. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Wright EA, Baron JA, Corbett KL, Nti AA, Malchau H, et al. Predictive value of Medicare claims data for identifying revision of index hip replacement was modest. J Clin Epidemiol. 2011 May;64(5):543–6. doi: 10.1016/j.jclinepi.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Rural-Urban Commuting Area Codes. Available at: http://depts.washington.edu/uwruca/index.php.
- 24.Sporer SM. How to do a revision total hip arthroplasty: revision of the acetabulum. Instr Course Lect. 2012;61:303–11. [PubMed] [Google Scholar]
- 25.Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011 Oct 5;93(19):1842–52. doi: 10.2106/JBJS.J.01197. [DOI] [PubMed] [Google Scholar]
- 26.Hartman CW, Garvin KL. Femoral fixation in revision total hip arthroplasty. J Bone Joint Surg Am. 2011 Dec 21;93(24):2311–22. doi: 10.2106/JBJS.9324icl. [DOI] [PubMed] [Google Scholar]
- 27.Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005 Mar;87(3):570–6. doi: 10.2106/JBJS.D.02121. [DOI] [PubMed] [Google Scholar]
- 28.Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010 Nov;468(11):3070–6. doi: 10.1007/s11999-010-1399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patil S, Garbuz DS, Greidanus NV, Masri BA, Duncan CP. Quality of life outcomes in revision vs primary total hip arthroplasty: a prospective cohort study. J Arthroplasty. 2008 Jun;23(4):550–3. doi: 10.1016/j.arth.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Lubbeke A, Katz JN, Perneger TV, Hoffmeyer P. Primary and revision hip arthroplasty: 5-year outcomes and influence of age and comorbidity. J Rheumatol. 2007 Feb;34(2):394–400. [PubMed] [Google Scholar]
- 31.Fender D, van der Meulen JH, Gregg PJ. Relationship between outcome and annual surgical experience for the charnley total hip replacement. Results from a regional hip register. J Bone Joint Surg Br. 2003 Mar;85(2):187–90. doi: 10.1302/0301-620x.85b2.12759. [DOI] [PubMed] [Google Scholar]
- 32.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001 Nov;83-A(11):1622–9. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Shervin N, Rubash HE, Katz JN. Orthopaedic procedure volume and patient outcomes: a systematic literature review. Clin Orthop Relat Res. 2007 Apr;457:35–41. doi: 10.1097/BLO.0b013e3180375514. [DOI] [PubMed] [Google Scholar]
- 34.Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. Early failures among 14,009 cemented and 1,326 uncemented prostheses for primary coxarthrosis. The Norwegian Arthroplasty Register, 1987–1992. Acta Orthop Scand. 1994 Feb;65(1):1–6. doi: 10.3109/17453679408993706. [DOI] [PubMed] [Google Scholar]
- 35.Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 36.Prokopetz JJ, Losina E, Bliss RL, Wright J, Baron JA, Katz JN. Risk factors for revision of primary total hip arthroplasty: a systematic review. BMC Musculoskelet Disord. 2012 Dec 15;13:251, 2474, 13–251. doi: 10.1186/1471-2474-13-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agabiti N, Picciotto S, Cesaroni G, Bisanti L, Forastiere F, Onorati R, et al. The influence of socioeconomic status on utilization and outcomes of elective total hip replacement: a multicity population-based longitudinal study. Int J Qual Health Care. 2007 Feb;19(1):37–44. doi: 10.1093/intqhc/mzl065. [DOI] [PubMed] [Google Scholar]
- 38.Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008 Sep;23(6 Suppl 1):9–14. doi: 10.1016/j.arth.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Martin CT, Callaghan JJ, Liu SS, Gao Y, Warth LC, Johnston RC. Disparity in total joint arthroplasty patient comorbidities, demographics, and postoperative outcomes based on insurance payer type. J Arthroplasty. 2012 Dec;27(10):1761, 1765.e1. doi: 10.1016/j.arth.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Warth LC, Callaghan JJ, Wells CW, Liu SS, Klaassen A, Gao Y, et al. Demographic and comorbid disparities based on payer type in a total joint arthroplasty cohort: implications in a changing health care arena. Iowa Orthop J. 2011;31:64–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987–99. J Bone Joint Surg Br. 2001 May;83(4):579–86. doi: 10.1302/0301-620x.83b4.11223. [DOI] [PubMed] [Google Scholar]
- 42.Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res (Hoboken) 2010 Apr;62(4):473–9. doi: 10.1002/acr.20036. [DOI] [PubMed] [Google Scholar]
- 43.Lenchik L, Sartoris DJ. Orthopedic aspects of metabolic bone disease. Orthop Clin North Am. 1998 Jan;29(1):103–34. doi: 10.1016/s0030-5898(05)70009-2. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi S, Saito N, Horiuchi H, Iorio R, Takaoka K. Poor bone quality or hip structure as risk factors affecting survival of total-hip arthroplasty. Lancet. 2000 Apr 29;355(9214):1499–504. doi: 10.1016/S0140-6736(00)02164-4. [DOI] [PubMed] [Google Scholar]
- 45.Rolfson O, Dahlberg LE, Nilsson JA, Malchau H, Garellick G. Variables determining outcome in total hip replacement surgery. J Bone Joint Surg Br. 2009 Feb;91(2):157–61. doi: 10.1302/0301-620X.91B2.20765. [DOI] [PubMed] [Google Scholar]
- 46.Soohoo NF, Farng E, Lieberman JR, Chambers L, Zingmond DS. Factors that predict short-term complication rates after total hip arthroplasty. Clin Orthop Relat Res. 2010 Sep;468(9):2363–71. doi: 10.1007/s11999-010-1354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavernia CJ, Contreras JS, Alcerro JC. Access to arthroplasty in South Florida. J Arthroplasty. 2012 Oct;27(9):1585–8. doi: 10.1016/j.arth.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Curtin B, Malkani A, Lau E, Kurtz S, Ong K. Revision after total knee arthroplasty and unicompartmental knee arthroplasty in the medicare population. J Arthroplasty. 2012 Sep;27(8):1480–6. doi: 10.1016/j.arth.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Khatod M, Inacio M, Paxton EW, Bini SA, Namba RS, Burchette RJ, et al. Knee replacement: epidemiology, outcomes, and trends in Southern California: 17,080 replacements from 1995 through 2004. Acta Orthop. 2008 Dec;79(6):812–9. doi: 10.1080/17453670810016902. [DOI] [PubMed] [Google Scholar]