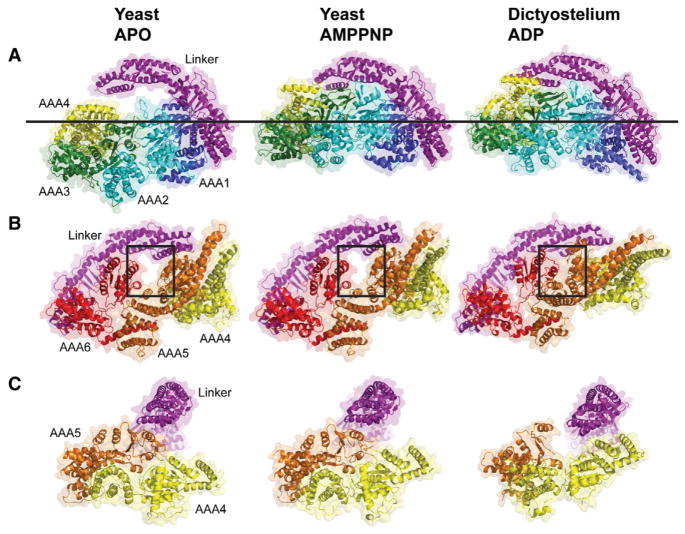
Figure 2. Comparison of the Motor Domain Ring between Yeast apo, Yeast AMPPNP, and Dictyostelium ADP Crystal Structures.
(A and B) Comparison of the two sides of the AAA ring in the indicated crystal structures. An upward movement of AAA2/3/4 toward the linker with the AMPPNP and ADP structures is observed (A), leading to a planar ring. In the ADP structure, AAA4 is lifted higher toward the linker. The line shows the common position of AAA1 in all structures. (B) An almost identical conformation of AAA5/6 for the apo and AMPPNP structures is observed, but the gap between AAA5 and AAA6 closes in the ADP structure (see box). Color coding of domains is the same as in Figure 1; structures are aligned on AAA1L.
(C) Movements of the large domains of AAA4 and AAA5 relative to the linker (linker subdomains 1,2 aligned in these structures). The linker is docked to AAA5L, and AAA5/6 are in similar states in the apo and AMPPNP structures. However, in the ADP structure, the linker is undocked as a result of a movement of AAA5. See Figure S2 for supporting information. PDBs: 4AKG (Schmidt et al., 2012) for yeast apo and 3VKG (Kon et al., 2012) for Dictyostelium ADP. Note: subdomain 0 of the linker, the AAA5 extension, and C sequence were removed from the Dictyostelium structure for comparison with yeast.