Figure 2.
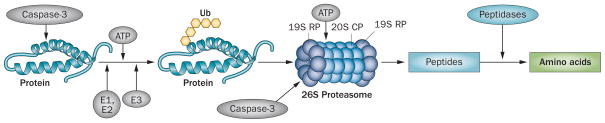
Chronic kidney disease-induced protein degradation by the UPS. An initial step in the degradation cascade involves cleavage of the complex structure of muscle protein by caspase-3, which produces substrates for degradation. Protein substrates are conjugated to Ub by an ATP-dependent process involving the enzymes E1, E2 and E3. The selectivity of protein substrates principally depends on recognition of the protein to be degraded by specific E3 Ub-ligases (for example, TRIM63 for muscle proteins). After five Ub proteins are attached to the protein substrate, the complex can be recognized by the 26S proteasome, which releases Ubs, unfolds the protein substrate and ‘injects’ it into the 20S CP in which proteins are degraded to peptides. At this stage, caspase-3 also cleaves the 26S protease regulatory subunit 4 and 26S protease regulatory subunit 8, which are specific subunit proteins of the 19S proteasome RP. This reaction stimulates degradation of proteins in the 20S proteasome CP. Peptides released into the cytoplasm are degraded into amino acids. Abbreviations: CP, core particle; RP, regulatory particle; Ub, ubiquitin; UPS, ubiquitin–proteasome system.
