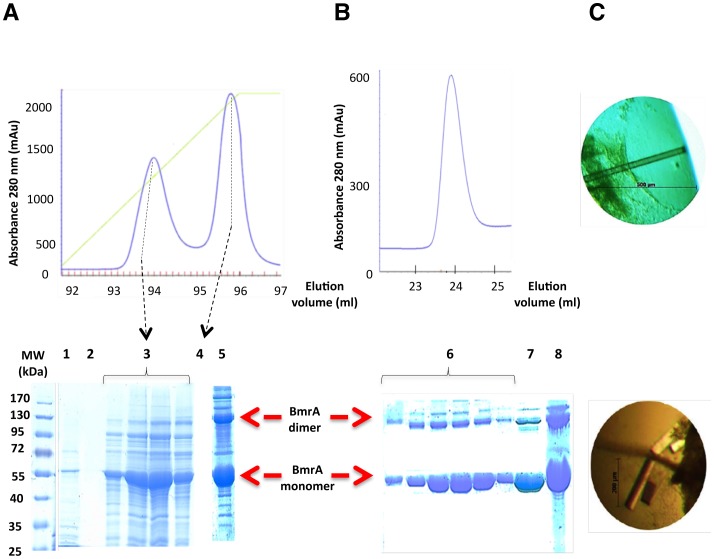
Figure 2. Purification and crystallization of BmrA protein in FC12.
A, elution of FC12 solubilized BmrA protein from a Q sepharose fast flow column. The absorbance of the protein was monitored at 280 nm and BmrA was eluted from the column with 600 mM NaCl. BmrA fractions were resolved on 10% SDS-PAGE. Lane 1, loading step. Lane 2, washing step. Lanes 3 and 4, protein eluted during the NaCl gradient. Lane 5, pool. Red arrows indicate the positions of the BmrA dimer and monomer. B, elution of BmrA from a Ni2+ high trap chelating column. SDS-PAGE, Lane 6, peak fractions. Lane 7, pool. Lane 8, concentrated pool. C, crystals obtained at 20°C by the vapor-diffusion hanging drop method with 2 µl of 10 mg/ml purified BmrA and 2 µl of the reservoir solution (18% polyethylene glycol 1500, 10% 2-methyl-2,4-pentanediol and 100 mM Tris-HCl pH 8.0).